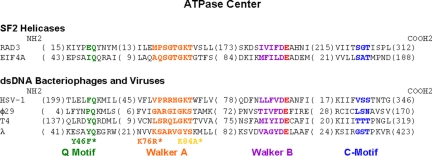
Fig. 1.
Sequence alignments (performed by Rao and coworkers, refs. 10 and 28) comparing the various functional domains of selected SF2 helicases and dsDNA packaging phage/virus motors. In color are the Q or adenine-binding motif (green), the Walker A motif (orange), the Walker B motif (purple), catalytic carboxylate (red), and the C motif (blue). Indicated with asterisks are mutants of phage λ's gpA examined in this study. In parentheses are the numbers of residues between adjacent domains indicated in the sequence alignments. According to Sun et al., structurally based alignments of functional domains of T4's gp17 crystal structure and nucleotide-binding domains of various other ATPases are mostly consistent with amino acid sequence alignments performed by Mitchell et al. (10, 23).