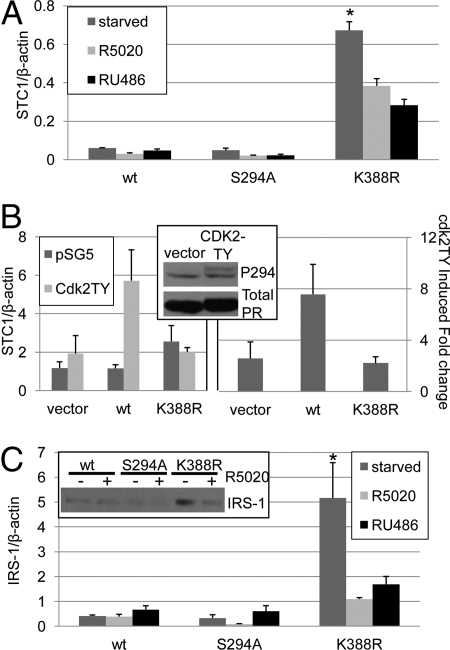
Fig. 2.
Phospho-dependent regulation of PR sumoylation alters target gene levels. (A) T47D cells stably expressing wt, S294A, or K388R PR-B were plated in triplicate cultures and serum starved or treated with R5020 (10−8M) or RU486 (10−7 M) for 48 h. Real-time PCR was performed and STC1 was normalized to β-actin (±SD, *P < 0.0001). (B) HeLa cells in triplicate cultures were transfected with vector, wt or K388R PR-B and either vector or CDK2TY. (Left panel) Real-time PCR was performed and STC1 was normalized to β-actin (±SD). (Right panel) Data from multiple experiments were combined and expressed as CDK2TY induced fold change over vector control (± SEM). (Inset) HeLa cells transfected with wt PR-B and either vector or CDK2-TY were starved for 24 h and Western blotted using phospho-S294 PR and total PR antibodies. (C) Triplicate cultures of T47D cells stably expressing wt, S294A, or K388R PR-B were serum starved or treated with R5020 (10−8 M) or RU486 (10−7 M) for 48 h, real-time PCR was performed, and IRS-1 was normalized to β-actin (±SD, *P < 0.01). (Inset) T47D cells stably expressing wt, S294A or K388R PR-B were serum starved for 24 h and treated with ethanol or R5020 (10−8 M) for 18 h then Western blotted for IRS-1.