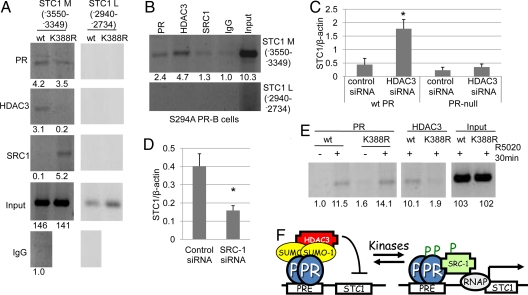
Fig. 3.
PR sumoylation alters the recruitment of cofactors to PR target promoters. (A) T47D cells stably expressing wt or K388R PR-B were serum starved for 48 h and subjected to ChIP assays using antibodies against PR, HDAC3, or SRC1(primers specific for the STC1 promoter). Normal rabbit IgG was used as an isotype control. (B) T47D cells stably expressing S294A PR-B were serum starved for 48 h and subjected to ChIP assays using antibodies against PR, HDAC3, or SRC1 or normal rabbit IgG and primers specific for the STC1 promoter. (C) PR-null HeLa cells and HeLa cells stably expressing wt PR-B were transfected with HDAC3 or negative control siRNA, and starved for 72 h, real-time PCR was performed, and STC1 was normalized to β-actin (±SD, *P < 0.006). (D) T47D cells stably expressing K388R PR-B were transfected with SRC1 or negative control siRNA, real-time PCR was performed, and STC1 was normalized to β-actin (±SD, *P < 0.005). (E) ChIP assays were performed in T47D cells stably expressing either wt or K388R PR-B using antibodies against PR and HDAC3 and primers specific for the HB-EGF promoter. Asterisk denotes statistical significance relative to cells transfected with control siRNA as determined by unpaired Student t tests. Clone 7 T47D K388R PR-B cells were used throughout these experiments. Bands on gels were quantified by densitometry (values are listed below each band). (F) High kinase activity in breast cancer cells blocks PR sumoylation and activates expression of growth promoting genes in the absence of progestins via altered cofactor recruitment.