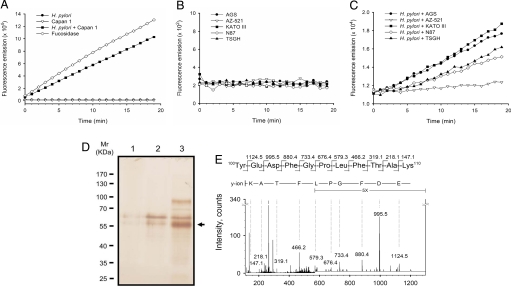
Fig. 2.
Activity assay, purification and identification of the secreted α-l-fucosidase from host cells co-cultured with H. pylori. Capan 1 (A) and 5 gastric adenocarcinoma cell lines (B and C) were examined, including AGS, AZ-521, KATO III, N87, and TSGH 9201. Before enzymatic activity measurement, cell culture media were subjected to affinity chromatography using a column packed with FNJ-immobilized Sepharose beads. (A) Measurement of α-l-fucosidase activity was carried out with H. pylori alone (filled circles), Capan 1 alone (inverted open triangles), or the coculture (filled squares) of Capan 1 and H. pylori (MOI ≈200:1). α-l-Fucosidase from Corynebacterium sp. was used as a positive control (open diamond). Furthermore, the activity of the secreted α-l-fucosidases by gastric cancer cell lines was measured either in the absence (B) or presence (C) of H. pylori (MOI ≈200:1). (D) The enzymatically active pooled fractions obtained from the affinity chromatography-based purification were analyzed by SDS/PAGE. The gel was stained with silver nitrate. Lane 1, H. pylori culture supernatant (≈2 × 1010 cells) was used for the purification. Lane 2, Capan 1 cell culture supernatant (≈1 × 108 cells). Lane 3, coculture supernatant of Capan 1 and H. pylori (MOI, ≈200:1). The predominant 55-kDa band, indicated by an arrow, was clearly visualized in Lane 3 as compared with Lanes 1 and 2. (E) Identification of α-l-fucosidase 2 (FUCA2). MS/MS spectrum of the signal [M + 2H]2+ at m/z 644.3 corresponded to the sequence 100YEDFGPLFTAK110 of FUCA2 (the second shaded sequence in Fig. S5). Only the most intense y-series fragment ions were labeled with single letter codes.