Fig. 3.
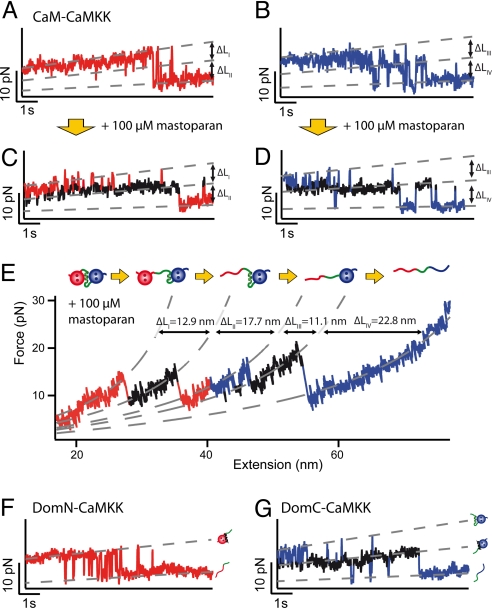
(A and B) Time traces of the unfolding regions of DomN (A) and DomC (B) of CaM-CaMKK, vpull = 1 nm/s. For both domains, a short-lived intermediate level appears. (C and D) Time traces of DomN (C) and DomC (D) of CaM-CaMKK at 100 μM mastoparan, vpull = 1 nm/s. The intermediate state (shown in black) is stabilized and can now be clearly distinguished from the two other levels. (E) Force vs. extension trace of CaM-CaMKK at 100 μM mastoparan, vpull = 10 nm/s and WLC fit curves (dashed gray lines). Above the trace, we show a scheme of the sequence of structural transitions as inferred from the increases in contour length. (F) Time trace of isolated DomN fused to CaMKK (DomN-CaMKK) at 10 μM mastoparan. No intermediate level can be detected, hence no sufficiently strong binding of CaMKK to isolated DomN takes place. (G) Time trace of isolated DomC fused to CaMKK (DomC-CaMKK) at 10 μM mastoparan, showing three levels (DomC folded, peptide-bound; DomC folded, peptide-unbound; DomC unfolded).