Abstract
AIMS
The aim of this study was to clarify the effects of CYP2D6 genotype on age-related change in flecainide metabolism in patients with supraventricular tachyarrhythmias. An in vitro study using microsomes was performed to identify other CYPs responsible for age-related change in flecainide metabolism.
METHODS
The study population comprised 111 genotyped patients: CYP2D6-homozygous extensive metabolizers (hom-EMs, n= 34), heterozygous EMs (het-EMs, n= 56), and intermediate and poor metabolizers (IMs/PMs, n= 21). Serum concentrations of flecainide and its metabolites [m-O-dealkylated flecainide (MODF) and m-O-dealkylated lactam of flecainide] were determined by use of a high-performance liquid chromatography. Metabolic ratio (MR) was expressed as serum concentrations of flecainide to its metabolites. In vitro formation of MODF was examined in human liver microsomes and cDNA-expressed CYP isoforms.
RESULTS
MR was higher in elderly patients (≥70 years) than in middle-aged patients (<70 years). The increase of MR in elderly patients differed among CYP2D6 genotypes: 1.6-fold in het-EMs [4.3, 95% confidence interval (CI) 2.8, 5.7 vs. 2.7, 95% CI 2.3, 3.1, P < 0.05], 1.5-fold in IMs/PMs (6.0, 95% CI 4.5, 7.6 vs. 4.1, 95% CI 2.9, 5.4, P < 0.05), and no change in hom-EMs. The in vitro study using microsomes revealed that both CYP2D6 and CYP1A2 were involved in the formation of MODF. MODF formation in CYP2D6 PM microsomes increased as CYP1A2 activity increased.
CONCLUSIONS
The results suggest that patients with poor CYP2D6-mediated metabolism (het-EMs and IMs/PMs) showed age-related reduction in flecainide metabolism because metabolism was taken over by CYP1A2, whose activity decreases with age.
Keywords: ageing, CYP1A2, CYP2D6, flecainide, metabolism
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
CYP2D6 is a main enzyme for flecainide metabolism in terms of the conversion of flecainide to m-O-dealkylated flecainide (MODF).
Age-related reduction of flecainide metabolism cannot be explained by CYP2D6 alone, the activity of which is known to be practically unchanged by ageing.
Flecainide metabolites including MODF have been found in plasma obtained from CYP2D6 poor metabolizers, suggesting that other CYPs may be involved in flecainide metabolism.
WHAT THIS STUDY ADDS
Age-related reduction in metabolic clearance of flecainide was remarkable in patients carrying CYP2D6 mutant alleles.
An in vitro study using human liver microsomes revealed that CYP1A2 was involved in MODF formation in addition to CYP2D6.
It is suggested that CYP1A2 plays an important role in flecainide metabolism in patients with poor CYP2D6-mediated metabolism.
Introduction
Flecainide acetate, a strong sodium channel blocker, is commonly used for a variety of supraventricular tachyarrhythmias [1, 2]. Flecainide is metabolized through conversion to m-O-dealkylated flecainide (MODF) and subsequent oxidation to m-O-dealkylated lactam (MODLF) [3, 4]. Impaired cytochrome P450 (CYP) 2D6 activity reduces flecainide clearance (CL/F) by 21% in intermediate metabolizers (IMs) and by 42% in poor metabolizers (PMs) [5, 6]. This suggests that the main enzyme that metabolizes flecainide is CYP2D6. Some reports, however, have suggested that other CYP isozymes are involved in flecainide metabolism, because MODF and MODLF can be detected in plasma even in CYP2D6 PMs [7–9], although the responsible enzymes have not yet been identified.
A population pharmacokinetic analysis revealed that flecainide CL/F was affected by age, sex, renal function, and genetic factors such as CYP2D6 genotype [6, 10]. The most important factor affecting flecainide metabolism in patients with normal kidney and liver function was ageing [6]. This age-related change in flecainide metabolism cannot be explained by the CYP2D6 pathway, because little change in CYP2D6 activity due to ageing has been reported [11–14]. Other CYP-mediated pathways are possibly involved in flecainide metabolism. A non-CYP2D6 pathway may provide different effects on age-related change of flecainide CL/F between CYP2D6 PMs and extensive metabolizers (EMs), because it is speculated to contribute more to flecainide metabolism in PMs than in EMs [8]. We therefore examined the effects of CYP2D6 genotype on age-related change in flecainide metabolism in patients with supraventricular tachyarrhythmias. We also performed an in vitro study using human liver microsomes and cDNA-expressed CYP isoforms to identify any other CYP-mediated pathway for flecainide metabolism.
Methods
Patients and sample collection
Patients treated with oral flecainide for supraventricular tachyarrhythmia were enrolled during an outpatient visit to our hospital (Table 1). Patients with any history of abnormal hepatic or renal function, smoking habit, or co-administration of CYP2D6 inhibitors (e.g. amiodarone, quinidine) or CYP1A2 inhibitors (e.g. fluvoxamine, ciprofloxacin) were excluded. Hepatic and renal function were normal in all enrolled patients (aspartate aminotransferase 24 ± 8 IU l−1; alanine aminotransferase 23 ± 10 IU l−1; serum creatinine 0.78 ± 0.16 mg dl−1; blood urea nitrogen 16 ± 4 mg dl−1). Renal function was also assessed using estimated glomerular filtration rate (eGFR) derived by the modified equation of abbreviated Modification of Diet in Renal Disease Study for Japanese [15]. The eGFR was 75 ± 26 ml min−1 per 1.73 m2. The patients had received oral flecainide (1.2–5.0 mg kg−1 day−1 as flecainide acetate) twice a day (n= 77), three times a day (n= 33), or four times a day (n= 1) for 1–91 months (mean 16.3 ± 20.0 months). Patients received other drugs as needed: digoxin, β-blockers (atenolol, carteolol, nadolol), Ca2+ antagonists (verapamil, diltiazem, nifedipine, amlodipine, nisoldipine, nicardipine), angiotensin-converting enzyme inhibitors (enalapril, imidapril, quinapril), anticoagulants (warfarin, aspirin, ticlopidine), H2-blockers (famotidine, ranitidine), or other drugs (lipid-decreasing drugs, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors).
Table 1.
Patients' characteristics
| Characteristic | Middle age | Elderly |
|---|---|---|
| Number of patients | 80 | 31 |
| Sex (male/female) | 65/15 | 21/10 |
| Age (year) | 55 ± 11 (53, 58) | 75 ± 4 (73, 76)* |
| Weight (kg) | 68 ± 10 (65, 70) | 60 ± 11 (56, 64)* |
| CYP2D6 genotype | ||
| Hom-EMs | 24 | 10 |
| *1/*1 | 16 | 4 |
| *1/*2 | 8 | 3 |
| *2/*2 | 0 | 3 |
| Het-EMs | 43 | 13 |
| *1/*5 | 2 | 1 |
| *1/*10 | 32 | 9 |
| *1/*21 | 1 | 0 |
| *2/*5 | 0 | 1 |
| *2/*10 | 8 | 2 |
| IMs/PMs | 13 | 8 |
| *5/*10 | 0 | 1 |
| *5/*36 | 0 | 1 |
| *10/*10 | 12 | 4 |
| *10/*21 | 0 | 1 |
| *10/*36 | 0 | 1 |
| *21/*36 | 1 | 0 |
| Flecainide daily dose (mg kg−1) | 2.4 ± 0.8 (2.3, 2.6) | 2.7 ± 0.9 (2.4, 3.0) |
| Serum conc. (ng ml−1) | ||
| Flecainide | 247 ± 108 (223, 271) | 391 ± 207 (318, 464)* |
| MODF | 59 ± 38 (50, 67) | 64 ± 34 (52, 76) |
| MODLF | 28 ± 20 (24, 33) | 28 ± 17 (22, 34) |
| Concentration-to-dose ratio | 105 ± 39 (97, 114) | 142 ± 50 (124, 159)* |
| Metabolic ratio | 2.8 ± 1.5 (2.5, 3.2) | 4.2 ± 2.6 (3.3, 5.1)* |
Significant difference between middle age and elderly was observed at P < 0.05. Results are presented as number or mean ± standard deviation (95% CI). Hom-EMs, homozygous extensive metabolizers; Het-EMs, heterozygous extensive metabolizers; IMs/PMs, intermediate and poor metabolizers; MODF, m-O-dealkylated flecainide; MODLF, m-O-dealkylated lactam of flecainide.
Because patients had received flecainide for at least 1 month, we assumed that steady-state levels of serum flecainide had been achieved. Blood was drawn to determine trough levels of flecainide and its metabolites between 08.30 and 11.00 h during an outpatient visit. On sampling days, patients postponed taking their morning flecainide until after blood drawing; the last dose was taken between 19.00 and 21.00 h the day before sampling. Serum samples separated from whole blood were stored at −20 °C until analysis.
The study was approved by the ethics committee of the University of Tsukuba, and written informed consent was obtained from all patients.
Genotyping of CYP2D6
Genomic DNA was isolated from peripheral blood with an extraction kit (Takara Bio, Shiga, Japan). CYP2D6*1, *2, *4, *5, *10, *14, *21, *36, and CYP2D6xN were determined by allele-specific polymerase chain reaction (PCR) with mismatch primers (ASPCR-MP) and step-down PCR. The PCR products were quantified by fluorescence detection with SYBR Green I (Takara) [16]. Single nucleotide polymorphism (SNP) typing kits for cytochrome P450 (STK-121, 122, 124, 125, 126 for ASPCR-MP; STK-123, 1277, 128 for step-down PCR) were obtained from Toyobo (Tokyo, Japan). Point mutations were detected with SNP typing kits and a GeneAmp PCR system 9700 (Applied Biosystems, Tokyo, Japan) in CYP2D6*2 (C2850T), *4 (G1846A), *10 (C100T), *14 (G1758A), *21 (2573 C insertion), CYP2D6*5 (CYP2D6 gene deletion), *36 (CYP2D6 gene conversion to CYP2D7P in exon 9), and CYP2D6xN (CYP2D6 gene duplication).
We designated CYP2D6*1 and *2 alleles as EM alleles, *10 as an IM allele, and *4, *5, *14, *21 and *36 as PM alleles and used these categories to assign the CYP2D6 genotype groups: homozygous EM (EM/EM: hom-EMs), heterozygous EM (EM/IM, EM/PM: het-EMs), and IM and PM (IM/IM, IM/PM, PM/PM: IMs/PMs).
Chemicals and microsomes
Flecainide acetate, MODF, MODLF, and an internal standard [N-(2-piperidinylmethyl)-2,3-bis(2,2,2-trifluoroethoxy)benzamide acetate] were kindly supplied by Eisai Co. (Tokyo, Japan). Furafylline, sulfaphenazole, ketoconazole, and nicotinamide adenine dinucleotide phosphate (NADPH)-regeneration system solution were obtained from BD Gentest (Woburn, MA, USA). Paroxetine hydrochloride and omeprazole were purchased from Wako Pure Chemicals (Osaka, Japan). Pooled microsomes from 50 human livers and single-donor microsomes (coded HH31, HH35 and HH61) of a CYP2D6 allelic variant (*4/*4) were obtained from BD Gentest. Marker enzyme activities and immunoquantified levels of individual CYPs were provided in the manufacturer's data sheets and supplied inserts. Microsomes from a baculovirus–insect cell line expressing CYP1A2, 2C9, 2C19, 2D6.1 (wild type), 2D6.10 (allelic variant), and 3A4 and control microsomes were purchased from BD Gentest.
In vitro study of flecainide metabolism
All experiments were carried out so that product formation increased linearly with incubation time and protein concentration. We confirmed that MODLF was not formed for 45 min incubation. After preincubation for 5–10 min, incubations were performed in duplicate at 37 °C in a shaking water bath for 30 min. Incubation mixtures contained microsome protein (1 mg ml−1), flecainide (0.5–1200 µM) and an NADPH-regeneration system [NADP (1.4 mM), glucose 6-phosphate (3.3 mM), MgCl2 (3.3 mM), glucose-6-phosphate dehydrogenase (2 U ml−1), final concentrations] in 200 µl of 100 mM potassium phosphate buffer (pH 7.4). The reaction was terminated by addition of 500 µl ice-cold acetonitrile. After addition of 50 µl internal standard (1 µg), the mixtures were vortexed and centrifuged at 8960 g for 5 min. The supernatant was evaporated to dryness under a stream of nitrogen at 45 °C. The residue was reconstituted with 100 µl mobile phase solution, and 40 µl was injected into a high-performance liquid chromatography (HPLC) system.
Chemical inhibition of MODF formation was examined by incubation of flecainide (50 µM) and pooled microsomes in the presence of CYP-selective inhibitors: furafylline (25 µM) for CYP1A2, sulfaphenazole (25 µM) for CYP2C9, omeprazole (10 µM) for CYP2C19, paroxetine (10 µM) for CYP2D6, and ketoconazole (5 µM) for CYP3A4. The 50 µM flecainide corresponded to the concentration in the liver under repeated administration of flecainide in clinical practice [17]. Mechanism-based inhibitors (furafylline and paroxetine) were preincubated with microsomes and the NADPH-regeneration system at 37 °C for 15 min. MODF formation was assessed in microsomes (20 pmol CYP) prepared from a baculovirus–insect cell line expressing CYP1A2, 2C9, 2C19, 2D6.1, 2D6.10 and 3A4. The incubations were conducted as above, except for CYP2D6.10 (60 pmol for 45 min).
Determination of serum flecainide and its metabolites in serum and microsomal incubation mixture
Flecainide, MODF and MODLF in serum and the microsomal incubation mixture were determined by HPLC on a conventional octadecylsilyl silica column and a fluorescence detector, as previously described [18]. Assay precision was evaluated by intra- and interday validation at 200 and 1000 ng ml−1 flecainide and at 30 and 200 ng ml−1 MODF and MODLF. The coefficients of variation for the intraday assay were 2.7–5.3% for flecainide, 3.0–4.2% for MODF, and 3.7–4.3% for MODLF. Those for the interday assay were 7.0–8.4%, 3.3–6.7% and 4.4–7.7%, respectively.
Data and kinetic calculations
The concentration-to-dose ratio (C/D) for flecainide was calculated as serum flecainide trough concentrations divided by dose per kilogram body weight. Metabolic ratio (MR) was calculated as:
in serum molar concentrations. We designated [MODF + MODLF] as the denominator of MR, because the MODLF is secondary metabolite of MODF.
Enzyme kinetic parameters for MODF formation were calculated using SigmaPlot (ver. 10.0; Systat Software, Richmond, CA, USA). Kinetic analyses were taken from an Eadie–Hofstee plot. The kinetics was described by one of two models:
enzyme Michaelis–Menten model: V=Vmax · S/(Km + S)
enzyme Michaelis–Menten model: V=Vmax1 · S/(Km1 + S) + Vmax2 · S / (Km2 + S),
where V is the velocity of the reaction at substrate concentration S, Vmax is the maximum velocity, and Km is the substrate concentration at which the reaction velocity is 50% of Vmax. Intrinsic clearance of the in vitro incubation was calculated as CLint=Vmax/Km.
Statistical analyses
Data are expressed as numbers, percentages, or means ± SD. Patient characteristics, C/D, and MR were compared between middle-aged and elderly patients by Student's t-test, Welch's t-test, or the Mann–Whitney U-test. The comparison of proportions was performed by use of the χ2 test, Fisher's exact probability test, or Mann–Whitney U-test. Multiple comparisons of C/D and MR among the three CYP2D6 genotype groups were performed using the Tukey–Kramer test following one-way anova or the Mann–Whitney U-test with Bonferroni's correction following the Kruskal–Wallis test. Correlation between variables was checked by calculating Spearman's rank correlation coefficient. P < 0.05 was considered significant.
Results
Age-related change in flecainide metabolism
The study population included 80 middle-aged (<70 years) and 31 elderly (≥70 years) patients (Table 1). The CYP2D6 allele frequencies of the patients were the same as previously reported [6, 10]. The distribution of CYP2D6 genotypes did not differ between age groups (χ2 test, P= 0.42; Table 1). The difference in eGFR between age groups was not statistically significant in the three genotype groups: hom-EMs (83 ± 34 vs. 67 ± 11 ml min−1 per 1.73 m2), het-EMs (78 ± 29 vs. 67 ± 12 ml min−1 per 1.73 m2) and IMs/PMs (70 ± 17 vs. 66 ± 16 ml min−1 per 1.73 m2). No difference in the male/female ratio was found between age groups (Table 2).
Table 2.
Effects of CYP2D6 genotype and age on concentration-to-dose ratio and metabolic ratio of flecainide
| Sex (M/F) | Concentration-to-dose ratio | Metabolic ratio | ||||
|---|---|---|---|---|---|---|
| CYP2D6 genotype groups | Middle aged | Elderly | Middle aged | Elderly | Middle aged | Elderly |
| Hom-EMs | 17/7 | 6/4 | 92 ± 35 | 115 ± 48 | 2.4 ± 0.9 | 2.5 ± 1.4 |
| (78, 106) | (85, 144) | (2.0, 2.7) | (1.7, 3.4) | |||
| Het-EMs | 36/7 | 10/3 | 104 ± 34 | 144 ± 45* | 2.7 ± 1.4 | 4.3 ± 2.7* |
| (94, 114) | (120, 169) | (2.3, 3.1) | (2.8, 5.7) | |||
| IMs/PMs | 12/1 | 5/3 | 133 ± 48† | 171 ± 48† | 4.1 ± 2.3† | 6.0 ± 2.2*† |
| (108, 159) | (138, 204) | (2.9, 5.4) | (4.5, 7.6) | |||
Significant difference between middle aged and elderly was observed at P < 0.05.
Significant difference between hom-EMs and IMs/PMs was observed at P < 0.05. Results are presented as number or mean ± standard deviation (95% CI). Hom-EMs, homozygous extensive metabolizers; Het-EMs, heterozygous extensive metabolizers; IMs/PMs, intermediate and poor metabolizers; M/F, male/female.
The C/D of flecainide was significantly higher in elderly than in middle-aged patients (P < 0.05, Table 1). The MR was also significantly higher in elderly patients (P < 0.05; Table 1). C/D and MR were significantly correlated (r= 0.49, P < 0.05; data not shown). Effects of CYP2D6 genotype on C/D and MR are presented in Table 2. The C/D and MR in IMs/PMs were significantly higher than those in hom-EMs (P < 0.05). There were no differences in C/D or MR between hom-EMs and het-EMs.
Age-related change in MR differed among CYP2D6 genotypes: MR increased exponentially in IMs/PMs and het-EMs but did not change in hom-EMs (Figure 1). There were significant differences in MR between elderly and middle-aged patients in IMs/PMs and het-EMs (P < 0.05), but not in hom-EMs (Table 2). The C/D of het-EMs was significantly higher in elderly than in middle-aged patients (P < 0.05; Table 2). The C/D of IMs/PMs and hom-EMs also were higher in elderly patients, although not significantly so (Table 2). Subgroup analysis with the data of male patients (n= 86) also showed similar results: significant difference in MR between elderly and middle-aged patients was observed in IMs/PMs (5.8 ± 1.6 vs. 4.0 ± 2.3, P < 0.05) and het-EMs (4.8 ± 2.8 vs. 2.6 ± 1.4, P < 0.05), but not in hom-EMs (2.1 ± 1.1 vs. 2.3 ± 1.0).
Figure 1.
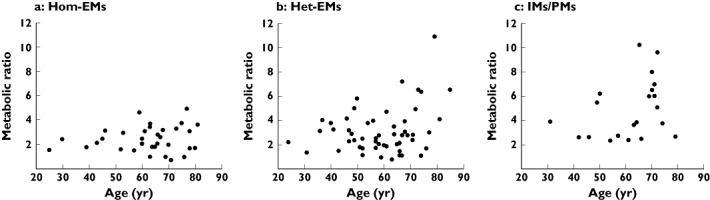
Relationships between age and flecainide metabolic ratio in (a) CYP2D6 homozygous extensive metabolizers (hom-EMs), (b) heterozygous extensive metabolizers (het-EMs), and (c) intermediate/poor metabolizers (IMs/PMs)
MODF formation in human liver microsomes and cDNA-expressed CYPs
A Michaelis–Menten plot and an Eadie–Hofstee plot supported a two-enzyme model and biphasic kinetics of MODF formation in pooled human liver microsomes (Figure 2). The estimated Km and Vmax of MODF formation are shown in Table 3. To identify the CYP isoforms responsible for MODF formation in the pooled microsomes, we examined the effects of specific CYP inhibitors on flecainide metabolism. Paroxetine and furafylline, potent inhibitors of CYP2D6 and CYP1A2, respectively, inhibited MODF formation by 61% and 64%. Other CYP inhibitors showed weak or no inhibitory effects on MODF formation (<10% inhibition; data not shown).
Figure 2.
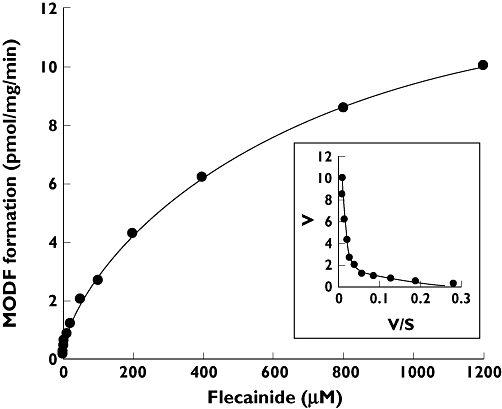
Kinetic profile of m-O-dealkylated flecainide (MODF) formation in pooled human liver microsomes with inset Eadie–Hofstee plot demonstrating biphasic kinetics. Data are the means of duplicate determinations
Table 3.
Kinetic parameters of MODF formation in human liver microsomes and cDNA-expressed CYPs
| Enzyme activity CYP2D6 (pmol min−1 mg−1) | CYP1A2 (pmol min−1 mg−1) | Km (µM) | Vmax (pmol min−1 mg−1 or nmol−1 CYP) | CLint (µl min−1 mg−1 or nmol−1 CYP) | |
|---|---|---|---|---|---|
| Pooled microsome | 81 | 540 | High-affinity component | ||
| 4.0 (0.7, 7.3) | 1.0 (0.7, 1.3) | 0.252 | |||
| Low-affinity component | |||||
| 673.1 (547.2, 799.0) | 14.0 (13.1, 14.9) | 0.021 | |||
| Microsomes containing cDNA-expressed CYP | |||||
| CYP1A2 | – | – | 587.9 (411.8, 764.0) | 34.4 (29.6, 39.2) | 0.058 |
| CYP2D6.1 | – | – | 2.8 (1.9, 3.6) | 19.5 (18.3, 20.7) | 7.063 |
| CYP2D6.10 | – | – | 8.2 (5.3, 11.0) | 2.9 (2.6, 3.1) | 0.352 |
| Single-donor microsome from CYP2D6 PMs | |||||
| HH31 | 4.1 | 2900 | 220.2 (206.9, 233.5) | 69.6 (68.2, 71.0) | 0.316 |
| HH35 | 0.4 | 240 | 504.5 (462.9, 546.1) | 8.2 (7.9, 8.5) | 0.016 |
| HH61 | 0.7 | 31 | 2186.4 (1653.4, 2719.3) | 6.0 (4.9, 7.0) | 0.003 |
Results are presented as value (95% CI). Enzyme activities of CYP2D6 and CYP1A2 are presented as activities of bufuralol 1′-hydroxylase and phenacetin O-deethylase, respectively. Vmax and CLint are expressed as per mg protein for human liver microsomes or per nmol CYP for microsomes containing cDNA-expressed CYP.
MODF was produced in microsomes containing cDNA-expressed CYP2D6.1 (18.0 pmol min−1 nmol−1 CYP) and CYP1A2 (3.1 pmol min−1 nmol−1 CYP). Other CYP isoforms showed no or weak activity of MODF formation (data not shown). The estimated Km and Vmax are shown in Table 3. CYP2D6.10 produced less MODF than CYP2D6.1 (CLint in Table 3). We examined the effect of CYP1A2 on MODF formation by using single-donor microsomes (coded HH31, HH35 and HH61) prepared from CYP2D6 PMs (genotyped as CYP2D6 *4/*4) with different CYP1A2 activities (Table 3). MODF formation increased as CYP1A2 activity increased (CLint in Table 3).
Discussion
We previously found that ageing was an important factor in estimating flecainide CL/F, which showed 30% reduction in elderly patients with normal kidney and liver functions [6]. This reduction might be due to age-related change in metabolic CL/F of flecainide, because the C/D was 1.4-fold higher and the MR was 1.5-fold higher in elderly patients than in middle-aged patients (Table 1). Another factor in the reduction of CL/F in the elderly may be age-related decline of GFR, even though the serum creatinine levels were normal. Although lower eGFR was found in elderly patients, the difference was not statistically significant between elderly and middle-aged patients.
It is particularly interesting that the age-related change in MR differed among CYP2D6 genotypes (Figure 1, Table 2). The enhancement of MR in elderly patients was found in het-EMs and IMs/PMs, but not in hom-EMs. Subgroup analysis with the male data supported this observation clearly. These findings suggest that carrying CYP2D6 mutant alleles causes an age-related decline in metabolic clearance of flecainide. However, this decline cannot be explained by a reduction in CYP2D6 activity, because several reports have suggested that CYP2D6 activity remains unchanged with ageing [11–14]. Our results, that CYP2D6 EMs showed no age-related decline in flecainide metabolism, support those previous findings.
We speculated that the age-related decline in flecainide metabolism was due to other CYPs responsible for the m-O-dealkylation of flecainide. MODF formation in pooled liver microsomes was mediated by enzymes with high and low affinity, which had low and high capacities, respectively (Figure 2, Table 3). Since selective chemical inhibitors of CYP2D6 (paroxetine) and CYP1A2 (furafylline) both decreased the formation of MODF, both CYP2D6 and CYP1A2 are involved in m-O-dealkylation of flecainide. Our experiments with cDNA-expressed CYP2D6 and CYP1A2 supported this hypothesis: CYP1A2, in which activity for MODF formation was only 1/6 that in CYP2D6, may be responsible for MODF formation in vivo, because the hepatic content of CYP1A2 is 8.5 times that of CYP2D6 [19].
We further confirmed the effect of CYP1A2 activity on MODF formation in microsomes prepared from three CYP2D6 PMs with different CYP1A2 activities. MODF formation in CYP2D6 PM microsomes increased as CYP1A2 activity increased (Table 3). This means that CYP1A2 is the main metabolic enzyme for the m-O-dealkylation of flecainide in CYP2D6 PMs. A similar situation may exist in the carriers of CYP2D6 IM and PM alleles, because MODF formation in microsomes expressing CYP2D6.10 was 1/20 that in CYP2D6.1. Our result that the MR of flecainide in CYP2D6 het-EMs and IMs/PMs increased with age may be due to a change in the main metabolic enzyme from CYP2D6 to CYP1A2, whose activity is known to decrease with ageing [11, 12, 20]. Age-related decline in CYP1A2 activity has been reported in several studies using CYP1A2 substrate such as theophylline and caffeine [11, 12, 20, 21].
Stereoselective metabolism of flecainide has been reported where the metabolic clearance of R-flecainide is lower than that of S-flecainide in CYP2D6 PMs [7–9]. This means that a non-CYP2D6 pathway, such as CYP1A2, may participate in the metabolism of S-flecainide predominantly.
Serum concentration is important in assessing the anti-arrhythmic effects and adverse effects of flecainide [22, 23]. It has been reported that the therapeutic range for serum flecainide was 300–1000 ng ml−1 in patients with supraventricular tachyarrhythmias: palpitation was not controlled in 65% of the patients with <300 ng ml−1, but in 11% with ≥300 ng ml−1[22]. Severe adverse events, such as ventricular arrhythmias, have been found in patients with serum flecainide >1000 ng ml−1[23]. Thus, dose adjustment of flecainide for keeping the therapeutic range is required for effective and safe use of this drug with large individual variations in the pharmacokinetics [22].
Flecainide is commonly used for atrial fibrillation [22, 24]. The prevalence and incidence of atrial fibrillation increase with age: among patients with a median age of 75 years, 84% are older than 65 years [25]. Therefore, understanding pharmacokinetics in the elderly is essential to providing tolerable effective therapy with flecainide. A long-term follow-up study of flecainide therapy revealed that the optimal maintenance dose of flecainide to control paroxysmal atrial fibrillation or flutter differed between middle-aged and elderly patients: a higher maintenance dose of flecainide (300 mg day−1) was required in half of patients <65 years old, but in only 6% of patients >65 years old [26]. This difference is due to age-dependent changes in the pharmacokinetics of flecainide. Our results further suggest that the age-dependent change of flecainide metabolism is due to the metabolic pathway via CYP1A2 in carriers with CYP2D6 mutant alleles. Thus, the CYP2D6 genotype of elderly patients should be useful for estimating the CL/F of flecainide.
Our study has several limitations for discussing the evidence that the age-dependent change of flecainide MR in CYP2D6 het-EMs and IMs/PMs was caused by switching the main metabolic enzyme from CYP2D6 to CYP1A2. First, we did not measure the in vivo CYP1A2 activity directly to confirm the age-dependent change of this enzyme activity. Second, the present study did not include patients carrying CYP2D6*4 representing PMs for Whites. It is therefore unknown whether or not similar observations in flecainide metabolism are obtained in Whites as in Asians.
In conclusion, we have identified the involvement of CYP1A2 in flecainide metabolism for the first time. This finding is consistent with a report that flecainide metabolism is enhanced by cigarette smoking [27], which induces CYP1A2. The patients with poor CYP2D6-mediated metabolism (het-EMs and IMs/PMs) showed an age-related reduction in flecainide metabolism, because metabolism was taken over by CYP1A2, whose activity decreases with age.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Encouragement of Scientists (No. 20926011) from the Japan Society for the Promotion of Science (for K.D.), and grant from Japan Research Foundation for Clinical Pharmacology (for M.H.). We greatly appreciate the assistance of Eisai Co. Ltd. for providing flecainide, its metabolites, and the internal standard for the assay. We acknowledge Ms K. Katori, Ms T. Edo, Mr T. Kawata, Ms S. Shimada and Ms H. Ohhata for their assistance.
Competing interests
None to declare.
REFERENCES
- 1.Crozier IG, Ikram H, Kenealy M, Levy L. Flecainide acetate for conversion of acute supraventricular tachycardia to sinus rhythm. Am J Cardiol. 1987;59:607–9. doi: 10.1016/0002-9149(87)91178-7. [DOI] [PubMed] [Google Scholar]
- 2.Kreeger RW, Hammill SC. New antiarrhythmic drugs: tocainide, mexiletine, flecainide, encainide, and amiodarone. Mayo Clin Proc. 1987;62:1033–50. doi: 10.1016/s0025-6196(12)65077-0. [DOI] [PubMed] [Google Scholar]
- 3.Conard GJ, Ober RE. Metabolism of flecainide. Am J Cardiol. 1984;53:41B–51B. doi: 10.1016/0002-9149(84)90501-0. [DOI] [PubMed] [Google Scholar]
- 4.McQuinn RL, Quarfoth GJ, Johnson JD, Banitt EH, Pathre SV, Chang SF, Ober RE, Conard GJ. Biotransformation and elimination of 14C-flecainide acetate in humans. Drug Metab Dispos. 1984;12:414–20. [PubMed] [Google Scholar]
- 5.Mikus G, Gross AS, Beckmann J, Hertrampf R, Gundert-Remy U, Eichelbaum M. The influence of the sparteine/debrisoquin phenotype on the disposition of flecainide. Clin Pharmacol Ther. 1989;45:562–7. doi: 10.1038/clpt.1989.73. [DOI] [PubMed] [Google Scholar]
- 6.Doki K, Homma M, Kuga K, Kusano K, Watanabe S, Yamaguchi I, Kohda Y. Effect of CYP2D6 genotype on flecainide pharmacokinetics in Japanese patients with supraventricular tachyarrhythmia. Eur J Clin Pharmacol. 2006;62:919–26. doi: 10.1007/s00228-006-0188-x. [DOI] [PubMed] [Google Scholar]
- 7.Gross AS, Mikus G, Fischer C, Hertrampf R, Gundert-Remy U, Eichelbaum M. Stereoselective disposition of flecainide in relation to the sparteine/debrisoquine metaboliser phenotype. Br J Clin Pharmacol. 1989;28:555–66. doi: 10.1111/j.1365-2125.1989.tb03542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross AS, Mikus G, Fischer C, Eichelbaum M. Polymorphic flecainide disposition under conditions of uncontrolled urine flow and pH. Eur J Clin Pharmacol. 1991;40:155–62. doi: 10.1007/BF00280070. [DOI] [PubMed] [Google Scholar]
- 9.Birgersdotter UM, Wong W, Turgeon J, Roden DM. Stereoselective genetically-determined interaction between chronic flecainide and quinidine in patients with arrhythmias. Br J Clin Pharmacol. 1992;33:275–80. doi: 10.1111/j.1365-2125.1992.tb04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doki K, Homma M, Kuga K, Aonuma K, Sakai S, Yamaguchi I, Kohda Y. Gender-associated differences in pharmacokinetics and anti-arrhythmic effects of flecainide in Japanese patients with supraventricular tachyarrhythmia. Eur J Clin Pharmacol. 2007;63:951–7. doi: 10.1007/s00228-007-0348-7. [DOI] [PubMed] [Google Scholar]
- 11.Kinirons MT, Crome P. Clinical pharmacokinetic considerations in the elderly. An update. Clin Pharmacokinet. 1997;33:302–12. doi: 10.2165/00003088-199733040-00005. [DOI] [PubMed] [Google Scholar]
- 12.Kinirons MT, O'Mahony MS. Drug metabolism and ageing. Br J Clin Pharmacol. 2004;57:540–4. doi: 10.1111/j.1365-2125.2004.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiner E, Bertilsson L, Säwe J, Bertling I, Sjöqvist F. Polymorphic debrisoquin hydroxylation in 757 Swedish subjects. Clin Pharmacol Ther. 1988;44:431–5. doi: 10.1038/clpt.1988.176. [DOI] [PubMed] [Google Scholar]
- 14.Pollock BG, Perel JM, Altieri LP, Kirshner M, Fasiczka AL, Houck PR, Reynolds CF., III Debrisoquine hydroxylation phenotyping in geriatric psychopharmacology. Psychopharmacol Bull. 1992;28:163–8. [PubMed] [Google Scholar]
- 15.Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis. 2007;50:927–37. doi: 10.1053/j.ajkd.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Ishiguro A, Kubota T, Soya Y, Sasaki H, Yagyu O, Takarada Y, Iga T. High-throughput detection of multiple genetic polymorphisms influencing drug metabolism with mismatch primers in allele-specific polymerase chain reaction. Anal Biochem. 2005;337:256–61. doi: 10.1016/j.ab.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Latini R, Cavalli A, Maggioni AP, Volpi A. Flecainide distribution in human tissues. Br J Clin Pharmacol. 1987;24:820–2. doi: 10.1111/j.1365-2125.1987.tb03252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doki K, Homma M, Kuga K, Watanabe S, Yamaguchi I, Kohda Y. Simultaneous determination of serum flecainide and its metabolites by using high performance liquid chromatography. J Pharm Biomed Anal. 2004;35:1307–12. doi: 10.1016/j.jpba.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Iwatsubo T, Hirota N, Ooie T, Suzuki H, Shimada N, Chiba K, Ishizaki T, Green CE, Tyson CA, Sugiyama Y. Prediction of in vivo drug metabolism in the human liver from in vitro metabolism data. Pharmacol Ther. 1997;73:147–71. doi: 10.1016/s0163-7258(96)00184-2. [DOI] [PubMed] [Google Scholar]
- 20.Loi CM, Parker BM, Cusack BJ, Vestal RE. Aging and drug interactions. III. Individual and combined effects of cimetidine and cimetidine and ciprofloxacin on theophylline metabolism in healthy male and female nonsmokers. J Pharmacol Exp Ther. 1997;280:627–37. [PubMed] [Google Scholar]
- 21.Chung WG, Kang JH, Park CS, Cho MH, Cha YN. Effect of age and smoking on in vivo CYP1A2, flavin-containing monooxygenase, and xanthine oxidase activities in Koreans: determination by caffeine metabolism. Clin Pharmacol Ther. 2000;67:258–66. doi: 10.1067/mcp.2000.104617. [DOI] [PubMed] [Google Scholar]
- 22.Homma M, Kuga K, Doki K, Katori K, Yamaguchi I, Sugibayashi K, Kohda Y. Assessment of serum flecainide trough levels in patients with tachyarrhythmia. J Pharm Pharmacol. 2005;57:47–51. doi: 10.1211/0022357055128. [DOI] [PubMed] [Google Scholar]
- 23.Morganroth J, Horowitz LN. Flecainide: its proarrhythmic effect and expected changes on the surface electrocardiogram. Am J Cardiol. 1984;53:89B–94B. doi: 10.1016/0002-9149(84)90509-5. [DOI] [PubMed] [Google Scholar]
- 24.Lafuente-Lafuente C, Mouly S, Longás-Tejero MA, Mahé I, Bergmann JF. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med. 2006;166:719–28. doi: 10.1001/archinte.166.7.719. [DOI] [PubMed] [Google Scholar]
- 25.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–8. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 26.Atarashi H. Long-term study of flecainide in patients with paroxysmal atrial fibrillation or flutter. J Clin Therap Med. 2007;23:841–57. [Google Scholar]
- 27.Holtzman JL, Weeks CE, Kvam DC, Berry DA, Mottonen L, Ekholm BP, Chang SF, Conard GJ. Identification of drug interactions by meta-analysis of premarketing trials: the effect of smoking on the pharmacokinetics and dosage requirements for flecainide acetate. Clin Pharmacol Ther. 1989;46:1–8. doi: 10.1038/clpt.1989.99. [DOI] [PubMed] [Google Scholar]


