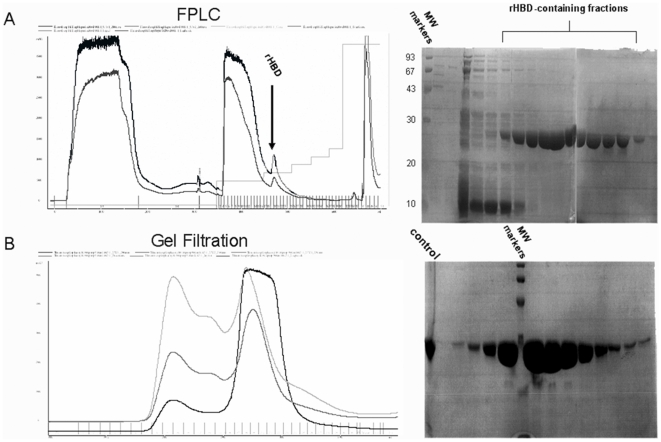
Figure 1. Cloning, expression and purification of the heparin binding domain (HBD) of thrombospondin-1.
A. Fast protein liquid chromatography (FPLC) of heparin-agarose purified rHBD. HBD constructs were transformed into competent E. coli, amplified, verified, and extracted for expression in expressing bacteria (Origami B), as described in Materials and Methods. Bacteria were lysed, and the rHBD was separated from the supernatant using FPLC equipped with a heparin-agarose column. The rHBD location was approximated using the FPLC fraction flow curve, as shown (left). The black line represents protein content of fractions eluted from the column. The peak containing the rHBD is marked. The dark gray line probably represents DNA remnants. SDS-PAGE served for rHBD identification. Clear, growing bands, located between 20–30 kDa, represent eluted rHBD. MW = molecular weight. Light gray line represents gradient B (elution salt gradient). B. Gel filtration. In order to obtain highly purified rHBD, FPLC protein-containing fractions were combined and separated using a molecular weight exclusion column. Protein content was determined using fraction-curve and SDS-PAGE. Black line = rHBD elution curve; Dark and light gray lines represent nonprotein side products. SDS-PAGE indicates the presence of a substantial amount of protein, located between 20–30 kDa. Control = rHBD; verified by mass spectrometry