In Her2/neu growth factor overexpressing poor prognosis breast cancers trastuzumab has shown to improve disease-free survival as well as overall survival in metastatic and primary diseases. On the basis of these positive results, trastuzumab has also been included into studies of neo-adjuvant therapy of Her2/neu-positive T2 tumors [1].
In our previous report on neo-adjuvant treatment, the initial reduction in circulating epithelial tumor cells (CETC) highly correlated to tumor size reduction [2] but further tumor shrinkage led to massive cell release [3].
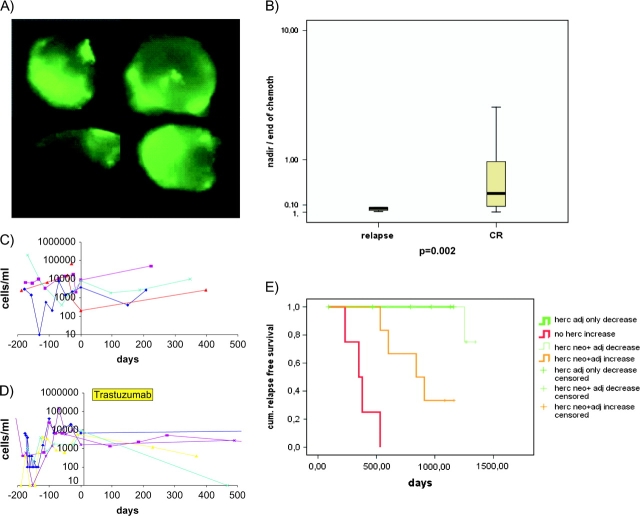
CETC from 26 Her2/neu-positive patients were further monitored during combined chemotherapy and antibody treatment [4] and during follow-up. Typical such cells are shown in Figure 1A.
Figure 1.
(A) Example of the procedure used for analysis of epithelial cells. The microscope scans a defined area on a slide to which a defined volume of cell suspension is applied. The majority of cells are normal blood cells, showing only background fluorescence. Positively stained green fluorescing cells. Cells in this window can be localized again viewed, photographed and reanalyzed. (B) Ratio between the lowest value of CETCs (nadir) and the CETC value at the end of therapy for patients with relapse and patients in CR (the lower the value the stronger the increase from nadir toward the end of therapy). (C and D) Course of numbers of CETC during neo-adjuvant chemotherapy and postsurgery (C) in 4 Her2/neu-positive patients withheld trastuzumab due to misclassification and (D) five of six patients receiving trastuzumab only after surgery. (E) Kaplan–Meier curves of relapse-free survival. Patients with Her2/neu-positive tumors without trastuzumab treatment exhibiting exclusively increasing CETC (bold red line), Her2/neu-positive tumors treated with trastuzumab with increasing CETC (bold orange line), Her2-positive tumors treated with trastuzumab with decreasing CETC (light green line) and patients with Her2/neu-positive tumors treated after surgery with stable or decreasing CETCs exclusively (bold green line).
Sixteen of the Her2/neu-positive patients were treated with four cycles of EC (epirubicine/cyclophosphamide) and four cycles of taxane (paclitaxel) together with trastuzumab and with trastuzumab for 1 year; thereafter. Six patients received trastuzumab only postoperatively and four patients did not receive trastuzumab at all.
In all, 5 of 14 (36%) assessable for tumor response at surgery showed pathological complete response (pCR) compared with only 5 of 30 (17%) Her2/neu-negative patients. Relapse-free survival until now is 65% versus 77%, respectively, thus relapses are still more frequent in spite of a higher frequency of pCR in Her2/neu-positive patients.
Patients who received taxane only showed a sharper increase in CETCs than patients treated with taxane/trastuzumab, the extent of increase being inversely correlated to the probability of relapse (Figure 1B) (P = 0.048).
During postsurgery observation, 4 of 4 patients who did receive trastuzumab neither before nor after surgery all had increasing CETC numbers (Figure 1C) and all have suffered relapse already within 1.5 years from diagnosis. From the trastuzumab neo-adjuvant arm, 9 of 15 assessable patients showed decreasing numbers of CETC during the following year of trastuzumab treatment and eight of nine of these patients have remained relapse free. Only one patient has suffered relapse in the brain. Of the 6 of 15 patients showing an increase in CETC in spite of trastuzumab treatment, four have suffered relapse (P = 0.015). Most surprisingly, six of six patients who received trastuzumab only after surgery all had decreasing CETC numbers (Figure 1D) and all are in sustained complete remission after up to 4 years. This difference is highly significant (P < 0.001) in the Kaplan–Meier analysis (Figure 1E).
In a Cox regression analysis, lack of response of CETC during anthracycline treatment and increasing CETC to subsequent trastuzumab treatment was highly predictive for relapse (P = 0.0001; hazard ratio = 17.756).
Thus, Her2/neu-positive tumors may release a chemotherapy surviving notably aggressive population of tumor cells which trastuzumab may eliminate and/or prevent from settling and growing into metastases or, with stable CETC numbers, may only silence [5] capable of regrowing after trastuzumab withdrawal.
The question arises if there is an interference between taxane and trastuzumab because patients receiving trastuzumab only after surgery all had left behind Her2/neu-positive trastuzumab responsive cells.
But even an excellent reduction of the Her2/neu-positve tumors during neo-adjuvant therapy does not prevent cells with a high metastatic capacity from being released.
Therefore, this treatment option should be reconsidered in these patients.
References
- 1.Coudert BP, Arnould L, Moreau L, et al. Pre-operative systemic (neo-adjuvant) therapy with trastuzumab and docetaxel for HER2-overexpressing stage II or III breast cancer: results of a multicenter phase II trial. Ann Oncol. 2006;17:409–414. doi: 10.1093/annonc/mdj096. [DOI] [PubMed] [Google Scholar]
- 2.Pachmann K, Camara O, Kavallaris A, et al. Quantification of the response of circulating epithelial cells (CEC) to neodadjuvant treatment of breast cancer: a new tool for therapy monitoring. Breast Cancer Res. 2005;7:R975–R979. doi: 10.1186/bcr1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camara O, Rengsberger M, Egbe A, et al. The relevance of circulating epithelial tumour cells (CETC) for therapy monitoring during neoadjuvant (primary systemic) chemotherapy in breast cancer. Ann Oncol. 2007;18:1484–1492. doi: 10.1093/annonc/mdm206. [DOI] [PubMed] [Google Scholar]
- 4.Lazaridis G, Pentheroudakis G, Pavlidis N. Integrating trastuzumab in the neoadjuvant treatment of primary breast cancer: accumulating evidence of efficacy, synergy and safety. Crit Rev Oncol Hematol. 2008;66:31–41. doi: 10.1016/j.critrevonc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Diermeier S, Horvath G, Knuechel-Clarke R, et al. Epidermal growth factor receptor coexpression modulates susceptibility to herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activation. Exp Cell Res. 2005;304:604–619. doi: 10.1016/j.yexcr.2004.12.008. [DOI] [PubMed] [Google Scholar]