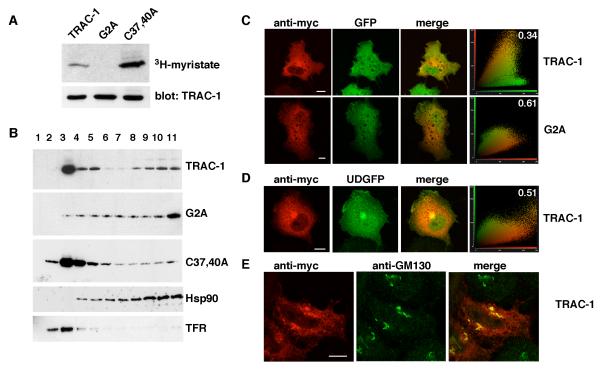
Figure 2. TRAC-1 is myristoylated and partially membrane-associated.
A. HEK293T cells were transiently transfected with TRAC-1/myc constructs and grown in the presence of 3H-myristic acid for 4 hours. TRAC-1/myc proteins were immunoprecipitated with anti-myc antibodies. One half of the sample was analyzed by SDS-PAGE and autoradiography (top), the other half by immunoblotting with anti-myc antibody (bottom).
B. HEK293T cells transfected with TRAC-1/myc constructs were homogenized in hypotonic buffer and fractionated on discontinuous 70%/65%/10% sucrose gradients. Fractions were collected from the top (fraction 1), and aliquots were analyzed by immunoblotting with anti-myc antibodies to detect TRAC-1 proteins, as well as with antibodies against Hsp90, a soluble protein, and Transferrin Receptor (TFR), a transmembrane protein.
C. COS-7 cells transfected with GFP and either TRAC-1/myc or G2A-TRAC-1/myc cDNAs were grown on 13 mm glass coverslips. 24 hours after transfection, cells were permeabilized, fixed, and stained with anti-myc as primary and rhodamine-conjugated goat-anti-mouse as secondary antibody. Confocal images of single channels as well as overlap images are shown. Scale bar: 10 μm. A cytofluorogram (right panels) is represented to show the intensities of red and green fluorophores per pixel. The relative amount of colocalization was determined using cross correlation analysis and the values (with 0 representing no colocalization, and 1 complete colocalization) are shown in the cytofluorgram.
D. COS-7 cells were transfected with TRAC-1/myc and a plasmid for UD-GFP, a myristoylated and palmitoylated protein that contains the unique domain of Lck fused to the N-terminus of GFP. Cells were stained, observed and analyzed as in (C). Scale bar: 10 μm.
E. HeLa cells were transfected with TRAC-1/myc and stained with anti-myc and anti-GM130, a cis-Golgi marker. Scale bar: 10 μm.