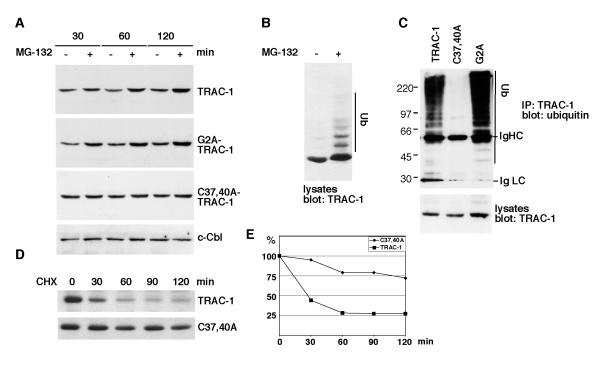
Figure 3. TRAC-1 is unstable as a result of auto-ubiquitination.
A. Jurkat T cells were transfected with the indicated TRAC-1/GFP constructs. The cells were either left untreated or were incubated with the proteasome inhibitor MG-132 (50 μM) for 30, 60 or 120 min. Equivalent amounts of cell lysates were analysed by Western blotting with anti-GFP to detect TRAC-1/GFP proteins, or with anti-c-Cbl.
B. HEK293T cells transfected with TRAC-1/myc cDNA were either left untreated or incubated with MG-132 (50 μM) for two hours. The cell lysates were blotted with anti-myc antibodies.
C. Plasmids for myc-tagged TRAC-1, C37,40A-TRAC-1 or G2A-TRAC-1 were cotransfected with HA-ubiquitin into HEK293T cells. TRAC-1 proteins were immunoprecipitated with anti-myc and blotted with anti-ubiquitin antibodies to detect ubiquitination (top). Lysates were blotted with anti-myc to detect the transfected TRAC-1 proteins (bottom).
D. COS-7 cells were transfected in suspension with myc-tagged TRAC-1 or C37,40A-TRAC-1 and equal cell numbers were divided over five dishes. Cells were incubated the next day with medium alone (0) or with medium containing cycloheximide (100 μg/ml) for the indicated times. Equal amounts of cell lysates were analyzed by Western blotting with anti-myc antibody.
E. Densitometry of the blots shown in D. For each time point, the density of the TRAC-1 band following subtraction of background levels and equalized for a loading control were determined. TRAC-1 levels are expressed as a percentage of levels in untreated cells.