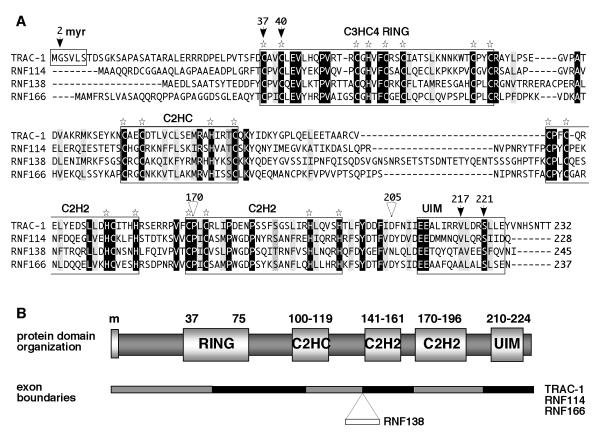
Figure 4. TRAC-1 is homologous to three other small C3HC4 RING proteins.
A. Amino acid alignments of the human proteins TRAC-1 (Q96EQ8), RNF114 (Q9Y508), RNF138 (Q8WVD3) and RNF166 (Q96A37). Alignments were performed using vector NTI software (Invitrogen). Identical residues are highlighted in black, conserved hydrophobic resides in light grey, other conservative changes in darker grey. Star symbols indicate the conserved Cys and His residues in the various domains. The arrows indicate amino acids in TRAC-1 that have been mutated in this study and the triangles show the positions where truncations have been made.
B. Schematic diagram of the domain composition of TRAC-1 and its family members. Indicated are the myristoylation site (m, present in TRAC-1 only) the RING domain, the newly identified C2HC and C2H2 zinc binding motifs and the UIM domain. The numbers refer to amino acid positions in TRAC-1. The gene composition with the exon boundaries relative to the protein domains is depicted below. RNF138 contains one extra exon compared to the other three proteins.