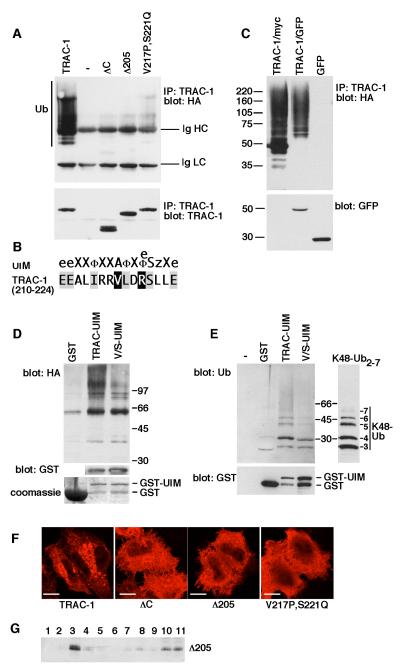
Figure 5. TRAC-1 contains a UIM-type ubiquitin-binding domain that is required for auto-ubiquitination.
A. HEK293T cells were transfected with plasmids expressing HA-ubiquitin and either myc-tagged wildtype TRAC-1, empty vector (-), TRAC-1ΔC, TRAC-1Δ205 or V217P,S221Q-TRAC-1. TRAC-1 proteins were immunoprecipitated with anti-myc and blotted with anti-HA to detect ubiquitination (top). Ub: ubiquitinated TRAC-1; Ig HC and Ig LC: the heavy chain (HC) and the light chain (LC) of the immunoprecipitating anti-myc antibody, respectively. The same blot was re-probed with anti-myc antibody to detect expression of TRAC-1 proteins (bottom).
B. Comparison of the consensus sequence for the UIM domain [according to [47]] with the TRAC-1 sequence of amino acids 210 - 224. The notation is as follows: e, negatively charged; X, helix favouring; Φ, hydrophobic, z, bulky hydrophobic or polar amino acid.
C. HEK293T cells were transfected with plasmids expressing HA-ubiquitin together with either TRAC-1/myc, TRAC-1/GFP or GFP. Proteins were immunoprecipitated with anti-myc (lane 1) or anti-GFP (lanes 2 and 3) and blotted with anti-HA to detect ubiquitination (top). The lysates were blotted for GFP (bottom).
D. GST fusion proteins were isolated on glutathione-sepharose beads and incubated with lysates from HA-ubiquitin transfected HEK293T cells. Beads were analysed by western blotting with anti-HA antibodies to detect the binding of ubiquitinated proteins to the recombinant proteins (top). TRAC-UIM: amino acids 181 – 232 of TRAC-1 fused to the C-terminus of GST; V/S-UIM: amino acids 181 – 232 with point mutations V217P and S221Q fused to the C-terminus of GST. GST itself was similarly purified and used as a control. To detect levels of expression for TRAC-UIM and V/S-UIM the blot was probed with anti-GST antibodies (middle). This is not shown for GST because of the high expression levels of this protein. The expression of GST, relative to the other constructs, is shown on a Coomassie stained gel (bottom).
E. GST, and GST fusion proteins as in (D) were isolated on glutathione beads and incubated with purified K48-linked poly-ubiquitin2-7 chains. Beads alone (-) were included as a control for specific binding. Beads were analysed for binding of ubiquitin with anti-ubiquitin antibodies (top). Input K48 poly-ubiquitin2-7 was analysed on the same polyacrylamide gel but a shorter exposure of the blot is shown. Beads were blotted with anti-GST antibodies to reveal relative levels of the proteins (bottom).
F. HeLa cells were transfected with constructs expressing myc-tagged wildtype TRAC-1, TRAC-1ΔC, TRAC-1Δ205 or V217P,S221Q-TRAC-1. 24 hours after transfection, cells were stained after with anti-myc antibodies and observed by confocal micrsocopy Scale bar: 10 μM.
G. HEK293T cells transfected with TRAC-1Δ205/myc were homogenized in hypotonic buffer and fractionated on discontinuous 70%/65%/10% sucrose gradients as in Fig. 2B. Fractions were collected from the top and aliquots were analyzed by immunoblotting with anti-myc antibodies. Membranes float up to the 65%/10% sucrose interface (fraction 3), whereas soluble proteins remain at the bottom (fractions 10 and 11).