Abstract
The pathogenesis of cardiac failure involves activation of the neurohumoral axis including stimulation of the sympathetic nervous system, the renin-angiotensin-aldosterone, and nonosmotic vasopressin systems. While these responses are critical in maintaining arterial pressure, they are associated with renal vasoconstriction, as well as sodium and water retention. In advanced circumstances, renal dysfunction and hyponatremia occur with cardiac failure. Even a modest rise in serum creatinine related to diminished renal function in heart failure patients is associated with increased risk for cardiovascular morbidity and mortality. Similarly, increased thirst and the nonosmotic stimulation of vasopressin in advanced cardiac failure leads to hyponatremia, which is also a major risk factor for mortality. Currently, V2 vasopressin receptor antagonists have been shown to correct hyponatremia in cardiac failure. One such agent, conivaptan, also is a V1 receptor antagonist which could theoretically benefit heart failure patients by decreasing cardiac afterload and remodeling. The effect of V2 receptor antagonists to correct hyponatremia in heart failure patients appears to be quite safe. However, to date no effect on mortality has been demonstrated.
Key Words: Hyponatremia, Antidiuretic hormone, Heart failure, Kidney function
Introduction
The level of renal function in patients with worsening heart failure is an important predictor of rehospitalization for cardiovascular events and death within 60 days [1]. Even mild deterioration of renal function is a risk factor for poor outcomes with congestive heart failure [2,3,4], myocardial infarction [5], and cardiovascular surgery [6]. Whether the diminished renal function in this cardiorenal syndrome is merely a marker or plays a pathogenetic role in the associated increased cardiovascular morbidity and mortality is an important issue [7]. An understanding of the pathogenesis of the sodium and water retention in cardiac failure patients provides insights into the pathogenetic role of the kidney in this cardiorenal syndrome. There have been recent classifications proposed to distinguish between cardiorenal and renocardiac syndromes depending on the initiating events [8,9,10,11].
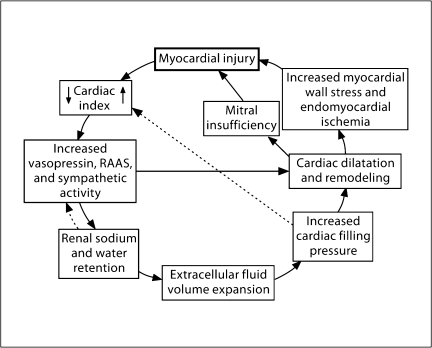
A decrease in cardiac output is known to activate the neurohumoral axis including stimulation of the sympathetic nervous system, the renin-angiotensin-aldosterone (RAAS), and nonosmotic vasopressin systems. In early cardiac failure, the renal sodium and water retention may return the cardiac index and the neurohumoral axis to levels indistinguishable from normal (fig. 1) [7]. However, with progression of cardiac failure, the plasma norepinephrine concentration and plasma renin activity correlate directly with increased mortality [12, 13]. Moreover, the arterial underfilling secondary to the decrease in stroke volume and cardiac index stimulates the baroreceptor-mediated, nonosmotic release of arginine vasopressin (AVP) as well as thirst. The resultant hyponatremia in cardiac failure has been shown to correlate with increased mortality [14]. With the development of the radioimmunoassay for AVP, cardiac failure patients were found to have increased plasma concentrations of AVP at decreased plasma osmolalities, which would suppress AVP secretion in normal subjects [15]. Further studies in cardiac failure patients demonstrated a negative correlation between plasma AVP and cardiac index; thus, the more severe the cardiac failure the higher the plasma AVP concentration [16].
Fig. 1.
Myocardial injury can lead to sodium and water retention, which can suppress the RAAS and return cardiac index to a normal range. The dashed lines indicate the pathways of these feedback mechanisms. To exclude myocardial injury when these feedback mechanisms have normalized the RAAS and cardiac index would be a mistake. Used with permission of Schrier [7].
AVP exerts short-term and long-term regulation of aquaporin 2 (AQP2) water channels in the principal cells of the collecting duct [17]. Short-term AVP regulation increases the trafficking of AQP2 to the apical membrane, thus increasing collecting duct water permeability. This increases urine concentration as electrolyte-free water moves from the lumen, across the collecting duct, into the interstitium and then into the circulation. AVP exerts a long-term regulation of urine concentration by increasing AQP2 protein expression. With AVP-related AQP2 exocytosis to the apical membrane of the principal cells, 3–6% of AQP2 spills into the lumen and is excreted in the urine. Studies in heart failure patients have shown that urinary AQP2 excretion is increased in cardiac failure patients as compared to control subjects. Moreover, New York Heart Association class III and IV patients had higher urinary AQP2 excretion rates than New York Heart Association class I and II patients [16].
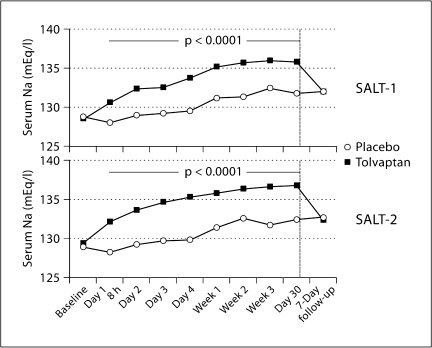
The role of arterial underfilling secondary to a decrease in cardiac index as the mediator of nonosmotic AVP release and water retention in cardiac failure patients has been examined. Patients with cardiac failure were treated with either an angiotensin-converting enzyme inhibitor or hydralazine to decrease cardiac afterload and increase cardiac index. The results of these clinical studies demonstrated improved urinary dilution and decreased plasma and platelet AVP in association with the increase in cardiac index [18]. Further incrimination of AVP in the water retention and hyponatremia of heart failure occurred when the nonpeptide, orally active antagonists of the vasopressin V2 receptor were discovered. V2 receptor antagonists have been administered to hyponatremic patients with cardiac failure and have resulted in increased solute-free water excretion and rise in plasma sodium concentration [19]. A decrease in urinary AQP2 excretion also occurred during administration of the V2 receptor antagonists in heart failure patients [20]. The randomized studies, SALT-1 and SALT-2, demonstrated a persistent 30-day rise in plasma sodium concentration in patients with cardiac failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone secretion with administration of the V2 receptor antagonist tolvaptan, which reversed on cessation of the agent (fig. 2) [20].
Fig. 2.
In both the SALT-1 and SALT-2 studies, the increase in the average daily area under the curve for serum sodium concentrations by visit from baseline to day 30 and 7-day follow-up was significantly greater in the tolvaptan group than in the placebo group. Used with permission of Szatalowicz et al. [15].
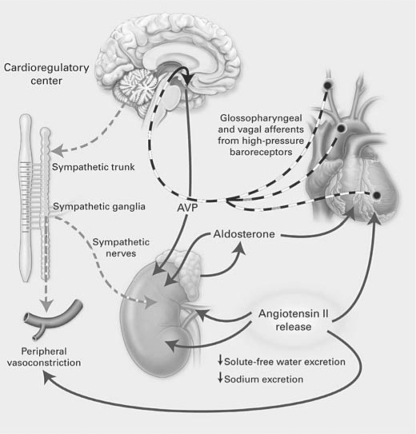
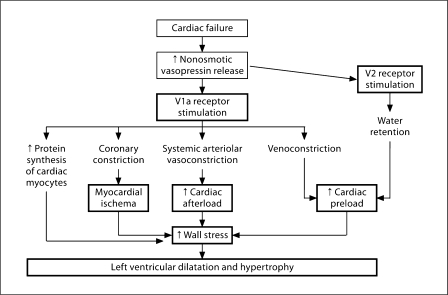
The pathophysiology of cardiac failure is summarized in figure 3, including unloading of the arterial baroreceptor secondary to the decrease in cardiac output with the resultant stimulation of the sympathetic nervous system, RAAS and vasopressin release [21]. An understanding of the pathophysiology of cardiac failure has led to prospective interventional studies (fig. 4) [22]. Studies with β-blockers [23], inhibitors of the RAAS [24, 25], and mineralocorticoid antagonists [26] have demonstrated an increase in survival in heart failure patients. While V2 receptor antagonists have been shown to improve electrolyte-free water reabsorption in heart failure patients, the randomized EVEREST study failed to demonstrate an effect on survival [27]. The FDA-approved AVP antagonist, conivaptan, for treating euvolemic and hypervolemic patients with hyponatremia is both a V1 and V2 receptor antagonist. The AVP stimulation of the V1 receptor is associated with vascular contraction, platelet aggregation, and proliferation of cardiac myocytes. In figure 5, the potential pathways are demonstrated whereby the combination of V1 and V2 receptor inhibition could have a salutary effect on patients with cardiac failure [7]. Such beneficial effects, however, remain to be demonstrated.
Fig. 3.
The pathophysiology of heart failure. Unloading of high-pressure baroreceptors (black circles) in the left ventricle, carotid sinus, and aortic arch generates afferent signals (dashed black lines) that stimulate cardioregulatory centers in the brain, resulting in the activation of efferent pathways in the sympathetic nervous system (dashed grey lines). The sympathetic nervous system appears to be the primary integrator of the neurohumoral vasoconstrictor response to arterial underfilling. Activation of renal sympathetic nerves stimulates the release of renin, thus activating the RAAS. Concomitantly, sympathetic stimulation of the supraoptic and paraventricular nuclei in the hypothalamus results in the nonosmotic release of AVP. Sympathetic activation also causes peripheral and renal vasoconstriction, as does angiotensin II. Angiotensin II constricts blood vessels and stimulates the release of aldosterone from the adrenal gland, and it also increases tubular sodium reabsorption and causes remodeling of cardiac myocytes. Aldosterone may also have direct cardiac effects on fibrosis, in addition to increasing the reabsorption of sodium and the secretion of potassium and hydrogen ions in the collecting duct. The solid black lines designate circulating hormones. Used with permission of Schrier and Abraham [21].
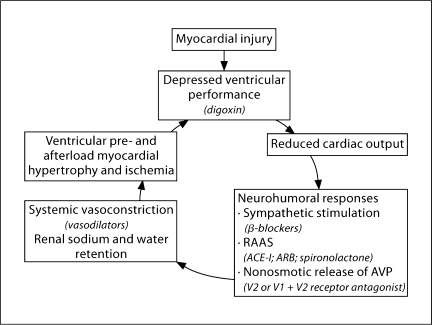
Fig. 4.
The vicious cycle of chronic congestive heart failure. Potential therapies are in parentheses. ACE-I = Angiotensin-converting enzyme inhibitors; ARB = angiotensin receptor blockers. Used with permission of Bichet et al. [18].
Fig. 5.
Pathways whereby vasopressin stimulation of V2 and V1a receptors can contribute to events that worsen cardiac function. Used with permission of Schrier [7].
A recent analysis of the OPTIME study [28] demonstrated that baseline and change in blood urea nitrogen during hospitalization of patients with acute decompensated cardiac failure was a better index of 60-day mortality than serum creatinine or estimated glomerular filtration rate. A potential explanation for this surprising finding relates to the effect of AVP on increased urea transporters and enhanced urea tubular reabsorption. Thus, the lower the cardiac output, the higher the plasma AVP will be with enhanced urea reabsorption and a rise in blood urea nitrogen [29].
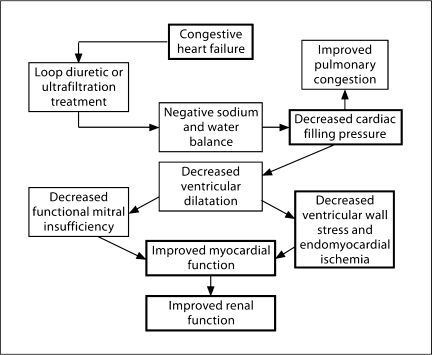
Removal of both sodium and water in cardiac failure patients is frequently needed and V2 antagonists only increase solute-free water excretion. Loop diuretics have been the primary therapy to treat pulmonary congestion and peripheral edema in patients with cardiac failure. Loop diuretics, however, block the sodium chloride transport in the macula densa of the nephron and thereby activate the RAAS with the potential deleterious effects on cardiac fibrosis and remodeling independent of changes in extracellular fluid volume. Ultrafiltration approaches are, therefore, under investigation, particularly in diuretic-resistant patients with heart failure. While excessive fluid removal in cardiac failure patients may decrease cardiac index and worsen renal function, there are potential benefits on cardiac function with fluid removal that are depicted in figure 6[7].
Fig. 6.
Mechanisms in congestive heart failure whereby negative sodium and water balance by loop diuretics or ultrafiltration therapy may improve myocardial and renal function. Used with permission of Schrier [7].
Acknowledgements
The authors would like to acknowledge the funding from the National Institutes of Health DK 19928 and the excellent editorial assistance of Jan Darling.
References
- 1.Gheorghiade M, Gattis W, Klein L. OPTIME in CHF trial: rethinking the use of inotropes in the management of worsening chronic heart failure resulting in hospitalization. Eur J Heart Fail. 2003;5:9–12. doi: 10.1016/s1388-9842(02)00178-2. [DOI] [PubMed] [Google Scholar]
- 2.Dries D, Exner D, Domanski M, Greenberg B, Stevenson L. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 3.The SOLVD Investigators Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 4.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM, Valsartan in Acute Myocardial Infarction Trial Investigators Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1839–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 6.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann L, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 7.Schrier RW. Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol. 2006;47:1–8. doi: 10.1016/j.jacc.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 8.Schrier RW. Cardiorenal versus renocardiac syndrome: is there a difference? Nat Clin Pract Nephrol. 2007;3:637. doi: 10.1038/ncpneph0673. [DOI] [PubMed] [Google Scholar]
- 9.Ronco C, House AA, Haapio M. Cardiorenal and renocardiac syndromes: the need for a comprehensive classification and consensus. Nat Clin Pract Nephrol. 2008;4:310–311. doi: 10.1038/ncpneph0803. [DOI] [PubMed] [Google Scholar]
- 10.Ronco C, House AA, Haapio M. Cardiorenal syndrome: refining the definition of a complex symbiosis gone wrong. Intensive Care Med. 2008;34:957–962. doi: 10.1007/s00134-008-1017-8. [DOI] [PubMed] [Google Scholar]
- 11.Ronco C, et al: Cardiorenal syndrome. J Am Coll Cardiol, in press. [DOI] [PubMed]
- 12.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma and norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 13.Francis GS, Cohn JN, Johnson G, Rector TS, Goldman S, Simon A. Plasma norepinephrine, plasma renin activity, and congestive heart failure. Relations to survival and the effects of therapy in V-HeFT II. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87(6 suppl):VI40–VI48. [PubMed] [Google Scholar]
- 14.Lee WH, Packer M. Prognostic importance of serum sodium concentration and its modification by converting-enzyme inhibition in patients with severe chronic heart failure. Circulation. 1986;73:257–267. doi: 10.1161/01.cir.73.2.257. [DOI] [PubMed] [Google Scholar]
- 15.Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet BG, Berl T, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305:263–266. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- 16.Funayama H, Nakamura T, Saito T, Yoshimura A, Saito M, Kawakami M, Ishikawa SE. Urinary excretion of aquaporin-2 water channel exaggerated dependent upon vasopressin in congestive heart failure. Kidney Int. 2004;66:1387–1392. doi: 10.1111/j.1523-1755.2004.00902.x. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper M. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 18.Bichet DG, Kortas C, Mettauer B, Manzini C, Marc-Aurele J, Rouleau JL, Schrier RW. Modulation of plasma and platelet vasopressin by cardiac function in patients with heart failure. Kidney Int. 1986;29:1188–1196. doi: 10.1038/ki.1986.126. [DOI] [PubMed] [Google Scholar]
- 19.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C, for the SALT Investigators Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 20.Martin PY, Abraham WT, Leiming X, Olson BR, Oren R, Ohara M, Schrier RW. Selective V2-receptor vasopressin antagonism decreases urinary aquaporin-2 excretion in patients with chronic heart failure. J Am Soc Nephrol. 1999;10:2165–2170. doi: 10.1681/ASN.V10102165. [DOI] [PubMed] [Google Scholar]
- 21.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 22.Schrier RW, Abdallah J, Weinberger H, Abraham W. Therapy of heart failure. Kidney Int. 2000;57:1418–1425. doi: 10.1046/j.1523-1755.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- 23.Daughty RN, Rodgers A, Sharpe N, MacMahon S. Effects of beta-blocker therapy in mortality in patients with heart failure: a systematic overview of randomized controlled trials. Eur Heart J. 1997;18:560–567. doi: 10.1093/oxfordjournals.eurheartj.a015297. [DOI] [PubMed] [Google Scholar]
- 24.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Geb H, Wong M. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of congestive heart failure. N Engl J Med. 1991;325:30–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 25.Garg R, Yusuf S, the Collaborative Group on ACE Inhibition Trials Overview of randomized trials of angiotensin-converting enzymes inhibitors in patients with heart failure. JAMA. 1995;273:1450–1457. [PubMed] [Google Scholar]
- 26.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 27.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) Investigators Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 28.Klein L, Massie B, Leimberger J, O'Connor C, Piña I, Adams I, Jr, Califf R, Gheorghiade M, for the OPTIME-CHF Investigators Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Circ Heart Fail. 2008;1:25–33. doi: 10.1161/CIRCHEARTFAILURE.107.746933. [DOI] [PubMed] [Google Scholar]
- 29.Schrier RW. Blood urea nitrogen (BUN) and serum creatinine: Not married in heart failure. Circ Heart Fail. 2008;1:2–5. doi: 10.1161/CIRCHEARTFAILURE.108.770834. [DOI] [PubMed] [Google Scholar]