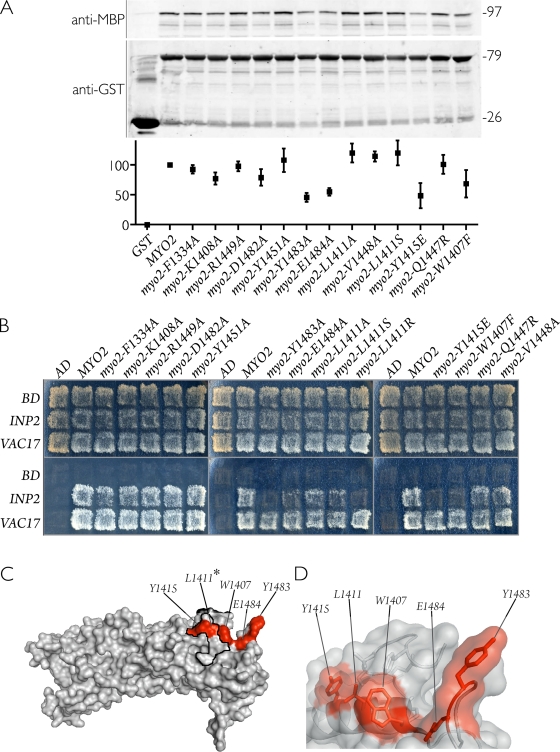
Figure 3.
Mutants of Myo2p defective in peroxisome inheritance display decreased affinity for Inp2p. (A) Glutathione Sepharose beads containing either GST fused to the cargo-binding tail of wild-type or mutant Myo2 proteins or GST alone were incubated with extracts of E. coli–synthesized MBP–Inp2p. (top) Bound MBP–Inp2p was analyzed by immunoblotting with anti-MBP antibodies. (middle) Total GST–Myo2p or GST protein levels were visualized by immunoblotting with anti-GST antibodies. Numbers at right denote the approximate sizes of proteins in kilodaltons. (bottom) Quantification of bound MBP–Inp2p, normalized to wild type and expressed as percent bound, was performed by densitometry. Graphic results show the means ± SEM of three independent experiments. (B) Myo2p point mutations specifically disrupt the ability of Myo2p to interact with Inp2p (peroxisomes) but not with Vac17p (vacuoles) in a yeast two-hybrid assay. Total growth of strains (top) and growth arising specifically from protein interaction (bottom) are shown. The pattern presented is representative of seven independent experiments. AD, activation domain; BD, binding domain. (C) Crystal structure of the Myo2p globular tail with the positions of point mutations that showed either weak or no interaction with Inp2p highlighted in red. The asterisk at L1411 indicates that the L1411R mutation was able to disrupt the Myo2p–Inp2p interaction in the yeast two-hybrid assay. The secretory vesicle–binding region is outlined in black. (D) Semitransparent surface view of the Myo2p structure overlaid on a ribbon diagram displaying the side chains of residues (red) implicated in binding Inp2p.