Figure 7.
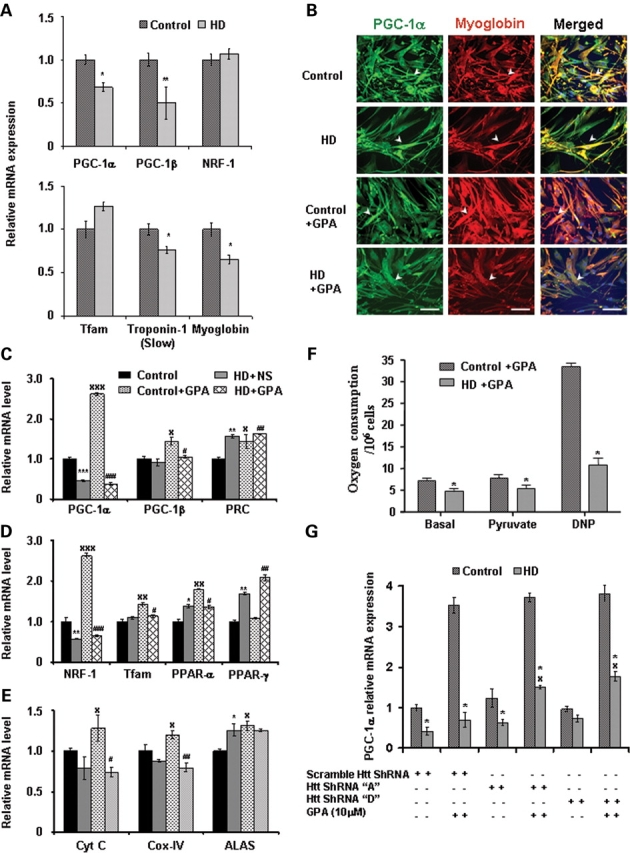
Impaired PGC-1α and target genes transcription, decreased oxidative capacity and cellular respiration in muscle biopsies and myoblasts from HD patients. (A) Quantitative real-time PCR analysis was performed in RNA isolated from muscle biopsies from symptomatic human HD patients and matched control subjects. Relative mRNA expression of PGC-1α and target genes and oxidative muscle markers (myoglobin and troponin I slow) was measured by normalizing values to β-actin. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01 (n = 9 control subjects and n = 13 HD patients). (B) We established muscle cell (myoblast) culture from HD patients and control subjects. To create chronic energy deprivation conditions, myoblast cultures were treated with GPA for 7 days. Immunofluorescence analysis of PGC-1α (green fluorescence), oxidative muscle marker myoglobin (red fluorescence) and DAPI (blue fluorescence) in myoblast cultures from HD patients and control subjects. Co-localization of PGC-1α and myoglobin is shown in merged images (yellow fluorescence). Arrowheads indicate immunoreactivity for PGC-1α and myoglobin. In HD myoblasts, decreased immunostaining and co-localization of PGC-1α and myoglobin is observed. Following GPA treatment, PGC-1α and myoglobin expression are increased in control myoblasts, but not in HD myoblasts. Scale bars = 50 µm. (C–E) Quantitative real-time PCR analysis was performed in RNA isolated from GPA-treated myoblast cultures from HD patients and control subjects. Relative mRNA expression of PGC-1α and target genes and mitochondrial function genes was measured by normalizing values to β-actin. Data are expressed as mean ± SEM. * and x = versus WT+NS, # = versus WT+GPA, *P < 0.05, **P < 0.01, ***P < 0.001 (n = 3 control subjects and n = 5 HD patients). (F) Measurement of cellular oxygen consumption in GPA-treated myoblast cultures from HD patients and control subjects. Oxygen consumption was recorded under basal conditions and after addition of pyruvate and the mitochondrial uncoupler dinitrophenol (DNP). Data are expressed as mean ± SEM. *P < 0.05 (n = 3 control subjects and n = 5 HD patients). (G) Increased PGC-1α mRNA expression in HD myoblasts by knockdown of mutant huntingtin. Control and HD myoblasts were transiently transfected with plasmid vector containing scramble and ShRNA target sequences A and D against mutant huntingtin. Knockdown of mutant huntingtin followed by GPA treatment caused a significant increase of PGC-1α mRNA expression in HD myoblasts. Data are expressed as mean ± SEM of two experiments. * = versus Control, x = versus scramble ShRNA. *, xP < 0.05.
