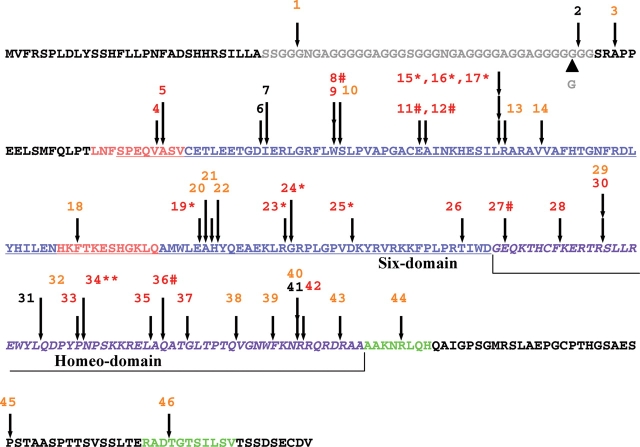
Figure 4.
Summary of structural alterations detected in the human SIX3 protein. A virtual translation of the human cDNA sequence used for our functional analysis is depicted with positions of variations according to individual mutation number (Table 1) and color representing activity in the overexpression assay: red = <50% activity, orange = 50–90% activity and black = >90% activity. An N-terminal poly-glycine segment (gray) is the most striking difference between multiple-species alignments and is present in human and rodent sequences but is largely absent in chick, zebrafish and Xenopus (data not shown). An uncommon length variant 205–207dupGGC adds an additional Glycine69 residue and was found in normal controls and can explain minor numbering differences among different SIX3 mutation reports. The canonical SIX domain (blue and underlined) also contains two eh1-like motifs (salmon-colored); note functional mutations were detected in both motifs. The homeodomain (purple and italic) is a target for in-frame deletions (e.g. 34**) and frameshift (e.g. 25*), whereas typical premature termination signals are indicated by the mutation number and the hash symbol. Note two putative C-terminal motifs (green) (38) are also functionally implicated by mutations leading to impaired activity.