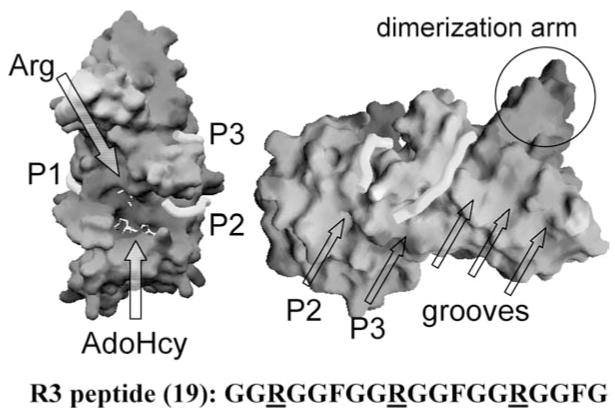
Figure 8.
Peptide-binding grooves (P1, P2, and P3) in the structure of ternary complex of PRMT1-AdoHcy-R3 peptide (sequence shown at the bottom): solvent-accessible molecular surface with bound AdoHcy and arginine shown as stick models and indicated by the arrows (left panel). If the central Arg-9 were the target bound in the active site, connecting peptide-binding sites P1 and P2 would cover the active site and the entire length of the peptide. When the end arginine (either Arg-3 or Arg-15) is bound in the active site, connection of peptide-binding sites P2 and P3 would account for the length of the whole peptide (right panel). Site P3 corresponds to one of the grooves perpendicular to the strands of the β-barrel domain.