Abstract
To gain further insights into the relationship between plasma phospholipid transfer protein (PLTP) and lipoprotein particles, PLTP mass and phospholipid transfer activity were measured, and their associations with the level and size of lipoprotein particles examined in 39 healthy adult subjects. No bivariate correlation was observed between PLTP activity and mass. PLTP activity was positively associated with cholesterol, triglyceride, apo B and VLDL particle level (rs=0.40–0.56, p≤0.01) while PLTP mass was positively associated with HDL-C, large HDL particles, and mean LDL and HDL particle sizes (rs=0.44–0.52, p<0.01). Importantly, plasma PLTP specific activity (SA) was significantly associated with specific lipoprotein classes, positively with VLDL, IDL, and small LDL particles (rs=0.42–0.62, p≤0.01) and inversely with large LDL, large HDL, and mean LDL and HDL particle size (rs=−0.42 to −0.70, p≤0.01). After controlling for triglyceride levels, the correlation between PLTP mass or SA and HDL size remained significant. In linear models, HDL size explained 45% of the variability of plasma PLTP SA while triglyceride explained 34% of the PLTP activity. Thus, in healthy adults a significant relationship exists between HDL size and plasma PLTP SA (rs=−0.70), implying that HDL particle size may modulate PLTP SA in the vascular compartment.
Keywords: Phospholipid Transfer Protein Specific Activity, PLTP Mass, PLTP Activity, LDL, HDL, Lipoprotein Particle Size
INTRODUCTION
Human plasma phospholipid transfer protein (PLTP) is a 476 amino acid protein secreted by a wide variety of tissues [1] and is associated predominantly with apo A-I-containing lipoproteins in plasma [2]. Among the diverse biological functions proposed for PLTP is its role in modulating vascular lipoproteins. In vitro, PLTP can facilitate the transfer of phospholipids and cholesterol from the lower-density lipoproteins to high-density lipoproteins [3–6]. It can mediate the conversion of HDL into larger and smaller particles [7, 8] and generate pre-β HDL in the process [9]. PLTP can also promote the efflux of cellular cholesterol and phospholipids from cholesterol-loaded cells [10, 11]. In mouse models, PLTP deficiency [12] and over-expression of PLTP [13] are associated with many lipoprotein abnormalities, emphasizing the importance of PLTP in lipoprotein metabolism. However, how much of the findings in genetically engineered mice are directly applicable to lipoprotein metabolism in man remains to be clarified. In contrast to mouse plasma, PLTP of low and high phospholipid transfer activity has been observed in human plasma [14–16]. The major goal of this study was to assess relationships between plasma PLTP and lipoprotein particles. To achieve this, PLTP mass and activity were measured in the plasma of healthy individuals and their associations with the level and size of lipoprotein particles examined. We report here that plasma PLTP specific activity (SA) is significantly correlated with the levels and mean size of the major classes of lipoprotein particles in healthy individuals, and that 45% of the variability of PLTP specific activity (SA) can be explained by HDL particle size. The implication of these observations to our understanding of the etiology and regulation of PLTP of low and high activity is discussed.
METHODS
Blood Samples
The subjects were participants of a study initially carried out to determine the relationships between cholesteryl ester transfer protein (CETP) and blood coagulability [17]. Thirty-nine healthy adult blood donors (19 men, 20 women) were recruited through the General Clinical Research Center’s blood donation program at The Scripps Research Institute. These volunteers were not taking any medications and were not current smokers. Furthermore, none of the women were on oral contraceptives or estrogen replacement therapy. Blood was drawn from routine vein puncture after an overnight fast and mixed with 0.129M sodium citrate (9:1). Plasma was promptly prepared by low-speed centrifugation, and stored at −80°C for lipid, lipoprotein, PLTP activity and PLTP mass measurements.
PLTP Activity
Plasma phospholipid transfer activity mediated by PLTP was determined by measuring the transfer of 14C-phosphatidycholine from phospholipid liposomes to HDL using an established radioassay [5]. Briefly, each assay tube contained 50µl HDL with 150nmol phospholipids, 50µl 14C-phosphatidycholine-labeled liposomes containing 50nmol phosphatidylcholine, 50µl of plasma samples pre-diluted 1:50 with Tris buffer (0.01mM Tris, 150 mM NaCl, 1mM EDTA, 0.01% sodium azide), and 250µl Tris buffer to bring the total assay volume to 400µl. Three separate dilutions of each plasma sample were assayed. All samples were incubated at 37°C for 15 min, a condition when the rate of transfer of phospholipids from liposomes to HDL is linear. Donor and acceptor particles were separated by precipitation with dextran sulfate and magnesium chloride [18]. Plasma PLTP activity was calculated as the percent of total radioactivity per assay tube transferred to HDL minus background transfer (tubes without plasma). Three plasma samples stored at −70°C until use, were included in each assay to control for inter-assay variation.
PLTP Mass
Plasma PLTP mass was measured using a new sandwich ELISA as follows: One hundred micro-liters of affinity-purified goat anti-PLTP antibody diluted to 5µg/ml in 0.1M carbonate buffer, pH9.6 were coated onto 96-well microplates (MaxiSorp™, Nunc) at 4°C overnight. The antibody-coated plates were washed three times with PBS buffer containing 0.05% Tween 20 (PBS-T) (Sigma). Excess binding surface on the wells were blocked with Starting Block (300µl/well) (Pierce) at room temperature (RT) for 2h. After washing the plates three times with PBS-T, 100µl of rPLTP (10–50ng/ml) or plasma samples (1:50) diluted with PBS-T were added to the wells and incubated at RT for 2h and then 4°C overnight. After washing, PLTP bound to the wells was detected using horseradish peroxidase-labeled affinity-purified chicken anti-PLTP antibodies with a 2h-incubation at RT, and the O-phenylenediamine dihydrochloride substrate (Sigma) with RT incubation for 15min. The color reaction product was monitored at 490nm, and the reaction with rPLTP was used to generate a standard curve to calculate plasma PLTP mass. Three serum samples stored at −70°C until use, were included in each assay to control for inter-assay variation, and duplicate dilutions of rPLTP and plasma samples were measured on each plate
The antibodies used in the ELISA were made by immunizing the host animals with purified full-length recombinant PLTP (rPLTP) that was produced in BHK-570 cells transfected with human PLTP-His tag cDNA, and purified from the serum-free conditioned medium of these cells with Ni-NTA agarose (Qiagen) (8). Specific goat and chicken anti-PLTP antibodies were isolated from goat and egg yolk immunoglobulins, respectively, by affinity chromatography using rPLTP covalently coupled to CNBr-activated Sepharose 4B according to the manufacturer’s protocol (GE Life Sciences). Horseradish peroxidase was conjugated to affinity purified chicken anti-PLTP antibodies using EZ-Link Plus Activated Peroxidase (Pierce). Affinity-purified goat and chicken anti-PLTP can inhibit essentially all phospholipid transfer mediated by rPLTP and by plasma PLTP. They also react with PLTP of low phospholipid transfer activity isolated from human plasma with the Mab4 monoclonal antibody [16].
Other Analytical Procedures
Plasma cholesterol, triglyceride, direct LDL and direct HDL cholesterol (C) were measured on a Hitachi Modular P Analytic System (Roche). Apolipoproteins (apo) A-I and B were measured with a nephelometer (Behring Diagnostics). These analyses were performed at the Northwest Lipid Metabolism and Diabetes Research Laboratories. Lipoprotein subclass particle concentrations and the average particle diameters of very low density lipoproteins (VLDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL) were measured by Nuclear Magnetic Resonance Spectroscopy (NMR) at LipoScience, Inc (Raleigh, NC). Data of nine lipoprotein subclasses based on particle diameter were used in this study. They were large VLDL (and chylomicrons if present) (> 60 nm), medium VLDL (35–60 nm), small VLDL (27–35 nm), intermediate lipoproteins (IDL) (23–27 nm), large LDL (21.2–23 nm), small LDL (18–21.2 nm), large HDL (8.8–13 nm), medium HDL (8.2–8.8 nm) and small HDL (7.3–8.2 nm) [18]. CETP mass was measured by a commercial ELISA (Wako Chemicals).
Statistical Analysis
Data were summarized by mean and standard deviation. To minimize any possible contribution of outliers, the Mann-Whitney U test was used for group comparisons, and Spearman rank order correlation analyses were used to determine the bivariate relationship between PLTP and lipoproteins particles. Linear regression analysis was used to examine predictors of plasma PLTP activity, mass, and specific activity. As the distribution of triglyceride, VLDL particles, and IDL particles were slightly skewed, they were logarithmically transformed prior to regression analyses. All analyses were carried out using SPSS statistical software. In consideration of multiple comparisons and multiple correlations, only P-values of ≤0.01 (two-tailed) in Mann-Whitney and Spearman analyses are reported as significant unless otherwise indicated.
RESULTS
Subject Characteristics
The 39 subjects in this study were between 19 and 56 years old with a mean age of 35.7 years, and a mean body mass index (BMI) of 26.2kg/m2. Sixty percent of the subjects were non-Hispanic white. As a group, their plasma lipid, apoA-I and apoB profiles were normal (Table 1). Nonetheless, a wide range of plasma cholesterol (123–243mg/dL), triglyceride (40–388mg/dL), LDL-C (54–166mg/dL), and HDL-C (26–70mg/dL) were seen with the men having significantly higher total triglyceride, and as expected, lower HDL-C than the women.
Table 1.
Characteristics of Study Participants
| All (n=39) | Women (n=20) | Men (n=19) | |
|---|---|---|---|
| Age (Yr.) | 35.7±7.2 | 36.4±7.0 | 35.0±7.5 |
| Body Mass Index (kg/m2) | 26.2±5.2 | 25.7±6.1 | 26.8±4.3 |
| Cholesterol (mg/dl) | 169±33.0 | 158±24.1 | 180±38.0 |
| Triglyceride (mg/dl) | 116±71.4 | 87.5±45.5 | 146±81.9* |
| LDL Cholesterol (mg/dl) | 98.2±28.8 | 89.3±22.7 | 108±32.0 |
| HDL Cholesterol (mg/dl) | 46.3±10.8 | 48.8±9.2 | 37.5±8.1* |
| Apo A-I (mg/dl) | 129±21.3 | 135±19.8 | 123±21.5 |
| Apo B (mg/dl) | 82.1±25.6 | 72.9±20.2 | 91.6±27.6 |
| CETP Mass (µg/ml) | 1.26±0.30 | 1.31±0.28 | 1.21±0.33 |
| PLTP Activity (µmol/ml/h) | 13.5±2.25 | 12.5±1.57 | 14.5±2.45 |
| PLTP Mass (µg/ml) | 1.55±0.28 | 1.60±0.20 | 1.49±0.34 |
| PLTP Specific Activity (µmol/µg/h) | 9.00±2.27 | 7.96±1.52 | 10.1±2.44** |
Numbers are mean ± S.D.
p<0.01, and
p<0.005 denote significant difference between men and women by Mann-Whitney U Test.
PLTP Activity and Mass
The mean plasma PLTP activity of these subjects was 13.5µmol/ml/h (range 9.1–18.9µmol/ml/h), and PLTP mass was 1.55µg/ml (range 0.67–2.06µg/ml), with a mean calculated SA of 9.0µmol/µg/h (range 5.6–17.5µmol/µg/h). Although there was no gender difference in PLTP mass and activity, men had significantly higher PLTP SA (p=0.002) (Table 1). No significant bivariate correlation was observed between plasma PLTP activity and PLTP mass.
Correlation of PLTP Parameters with Subject Characteristics
There was no correlation between any of the PLTP parameters and age or CETP mass. However, plasma PLTP activity was significantly associated with BMI, cholesterol, triglyceride, and apo B (rs=0.399–0.563, p≤0.01 to ≤0.001) (Table 2), and plasma PLTP mass was significantly associated with HDL-C (rs=0.438, p=0.005). Plasma PLTP SA was positively associated with cholesterol (rs=0.398, p=0.01), triglyceride (rs=0.618, p<0.001) and apo B (rs=0.447, p=0.004) but inversely associated with HDL-C (rs=−0.471, p=0.003). No significant correlation was observed between CETP mass and the parameters in Table 1.
Table 2.
Bivariate and Partial Correlations between PLTP and Characteristics of Study Participants
| Subject Characteristics | PLTP Activity | PLTP Mass | PLTP SA |
|---|---|---|---|
| Spearman Correlation Coefficient | |||
| Age | 0.302 | −0.066 | 0.151 |
| Body Mass Index | 0.463** | −0.297 | 0.439* |
| CETP mass | 0.090 | −0.226 | 0.237 |
| Cholesterol | 0.500*** | −0.171 | 0.398* |
| Triglyceride | 0.563*** | −0.329 | 0.618*** |
| LDL Cholesterol | 0.339 | −0.234 | 0.382 |
| HDL Cholesterol | −0.106 | 0.438** | 0.471** |
| Apo A-I (mg/dl) | 0.069 | 0.195 | −0.197 |
| Apo B (mg/dl) | 0.399* | −0.290 | 0.447** |
| Partial Correlation Coefficient Controlling for Triglyceride | |||
| Body Mass Index | 0.131 | −0.076 | 0.025 |
| Cholesterol | 0.242 | 0.253 | −0.199 |
| LDL Cholesterol | 0.126 | 0.076 | −0.074 |
| HDL Cholesterol | 0.178 | 0.432* | −0.375 |
| Apo A-I (mg/dl) | 0.073 | 0.362 | −0.371 |
| Apo B (mg/dl) | 0.073 | 0.079 | −0.109 |
p<0.01
p<0.005
p<0.001 denote significant correlation between PLTP and lipoproteins
Of all the correlations observed in Table 2, the strongest relationship was between PLTP activity or SA and plasma triglyceride. As plasma triglyceride was also significantly correlated with total cholesterol, LDL and HDL cholesterol, apo B, and BMI (rs between 0.459 and 0.773, p<0.005), partial correlations holding triglyceride constant was carried out to determine its effect on the bivariate correlations in Table 2. Interestingly, controlling for triglyceride erased all the significant associations between plasma PLTP activity or SA with total cholesterol, LDL-C, apo B, and BMI (Table 2) but greatly increased the correlation coefficient between PLTP activity and mass from 0.106 (p=0.522) to 0.419 (p=0.009). Importantly, the correlation between HDL cholesterol and PLTP mass or PLTP SA remained significant after adjustment for triglyceride (rs=432, p=0.007 and rs=0.375, p=0.02, respectively). Thus, the correlation of PLTP activity or PLTP SA with BMI, cholesterol, and apo B was primarily due to their mutual association with triglyceride, but the correlation of PLTP mass or SA with HDL-C was largely independent of triglyceride.
Lipoprotein Particles
The NMR lipoprotein particle profiles were studied in 35 of the 39 subjects, and the data are presented in Table 3. In men, the higher plasma triglyceride was reflected in having significantly more VLDL particles. In women, the higher HDL-C level was due to the increased presence of large HDL particles. Although LDL-C was not different between men and women, men had significantly fewer large LDL and significantly more small LDL particles. As a result of the gender difference in the proportion of large and small LDL and HDL particles, the mean size of LDL and HDL particles was smaller in men.
Table 3.
NMR Lipoprotein Profile of Study Participants
| Lipoproteins Particles | All (n=35) | Women (n=18) | Men (n=17) |
|---|---|---|---|
| Particle Concentration | |||
| VLDL (Large) (nmol/L) | 3.7±4.9 | 1.7±2.8 | 5.9±5.8** |
| VLDL (Medium) (nmol/L) | 27.3±21.5 | 16.3±14.9 | 39.0±21.6*** |
| VLDL (Small) (nmol/L) | 41.8±21.1 | 36.0±14.7 | 47.9±25.2 |
| IDL (nmol/L) | 43.6±47.5 | 27.3±32.4 | 60.8±55.4 |
| LDL (Large) (nmol/L) | 362±199 | 454±175 | 263±179** |
| LDL (Small) (nmol/L) | 786±441 | 529±322 | 1058±387*** |
| HDL (Large) (μmol/L) | 6.2±3.8 | 8.4±3.0 | 3.9±3.2*** |
| HDL (Medium) (µmol/L) | 6.5±4.9 | 6.5±5.3 | 6.6±4.7 |
| HDL (Small) (µmol/L) | 19.9±5.7 | 18.4±4.7 | 21.6±6.3 |
| Mean Particle Size | |||
| VLDL size (nm) | 50.7±8.0 | 49.2±7.2 | 52.2±8.8 |
| LDL size (nm) | 20.9±0.9 | 21.4±0.7 | 20.3±0.7*** |
| HDL size (nm) | 8.8±0.4 | 9.1±0.3 | 8.6±0.3*** |
Numbers are mean ± S.D.
p<0.005, and
p<0.001 denote significant difference between men and women by Mann-Whitney U Test.
PLTP and Lipoprotein Particles
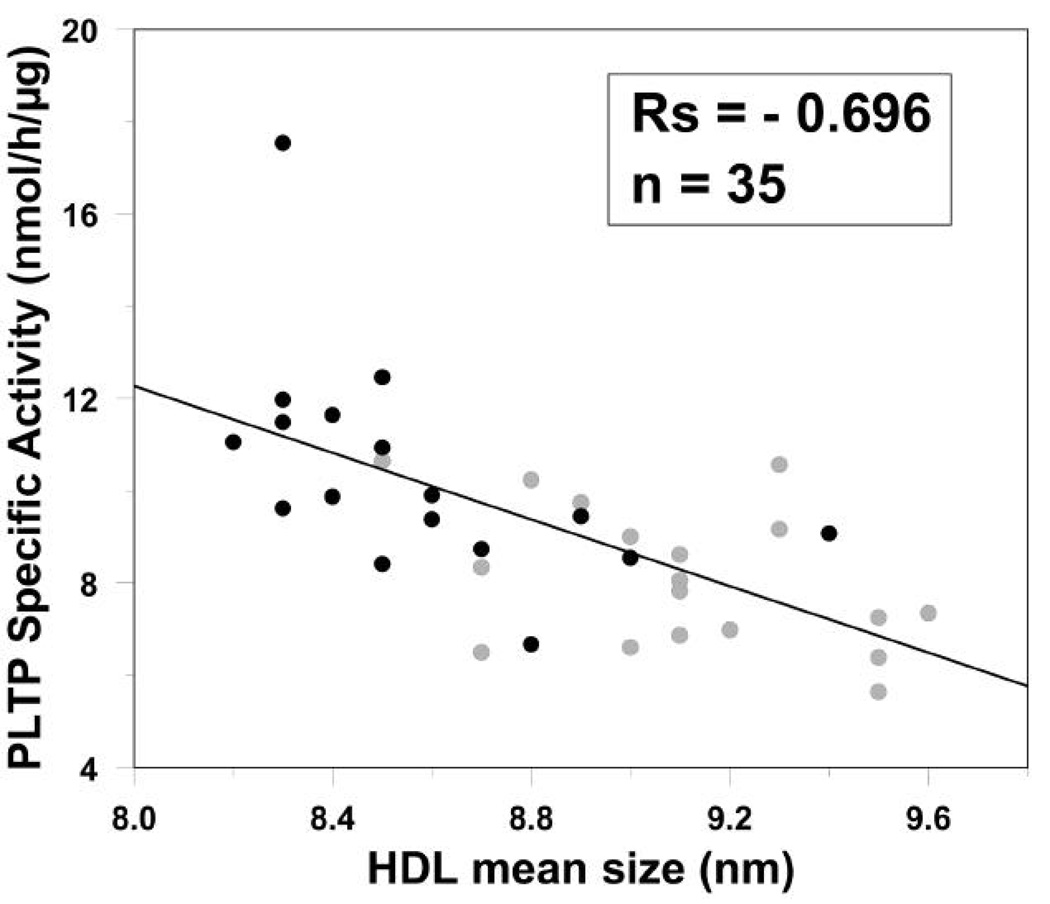
Bivariate correlations between plasma PLTP parameters and lipoprotein particles are shown in Table 4. Plasma PLTP activity was most strongly associated with large VLDL (rs=0.547, p<0.001), and PLTP mass was significantly associated with large HDL particles (rs=0.477, p=0.004), mean HDL size (rs=0.516, p=0.002) and mean LDL size (rs=0.450, p=0.007). Significant correlations were observed between PLTP SA and select lipoprotein subclasses (Table 4). PLTP SA was positively correlated with the levels of large VLDL (rs=0.551, p<0.001) but negatively correlated with large HDL (rs=−0.584, p<0.001). The strongest correlation was observed between PLTP SA and HDL particle size (rs=−0.696, p<0.001) (Fig. 1). Controlling for triglyceride in partial correlation analysis greatly weakened the associations between PLTP parameters and non-HDL particles. In contrast, the correlations of PLTP mass and PLTP SA with HDL size remained significant after triglyceride adjustment (rs=0.450 and −0.531, p=0.008 and 0.001), and the correlation with large HDL was only slightly weakened (rs=0.401 and −0.410, p=0.019 and 0.016).
Table 4.
Bivariate and Partial Correlations between PLTP and Lipoprotein Particles
| Lipoprotein Particles | PLTP Activity | PLTP Mass | PLTP SA |
|---|---|---|---|
| Spearman Correlation Coefficient | |||
| Particle Concentration | |||
| VLDL (Large) | 0.547*** | −0.296 | 0.551*** |
| VLDL (Medium) | 0.399 | −0.111 | 0.424* |
| VLDL (Small) | 0.339 | 0.093 | 0.151 |
| IDL | 0.354 | −0.403 | 0.492** |
| LDL (Large) | −0.054 | 0.397 | −0.415* |
| LDL (Small) | 0.308 | −0.370 | 0.526** |
| HDL (Large) | −0.241 | 0.477** | −0.584*** |
| HDL (Medium) | 0.126 | −0.010 | 0.034 |
| HDL (Small) | 0.249 | −0.300 | 0.345 |
| Mean Particle Size | |||
| VLDL size | 0.349 | −0.205 | 0.281 |
| LDL size | −0.182 | 0.450* | −0.509** |
| HDL size | −0.324 | 0.516** | −0.696*** |
| Partial Correlation Coefficient Controlling for Triglyceride | |||
| VLDL (Large) | 0.063 | −0.218 | 0.164 |
| VLDL (Medium) | 0.073 | 0.116 | −0.010 |
| IDL | 0.090 | −0.094 | 0.141 |
| LDL (Large) | 0.058 | 0.302 | −0.366 |
| LDL (Small) | 0.148 | −0.153 | 0.209 |
| HDL (Large) | 0.040 | 0.401 | −0.410 |
| LDL size | 0.015 | 0.348 | 0.336 |
| HDL size | −0.063 | 0.450* | −0.531*** |
p<0.01
p<0.005
p<0.001 denote significant correlation between PLTP and lipoproteins.
Figure 1.
Correlation of HDL mean size with PLTP specific activity. Males: black circles; Females: grey circles.
No correlation was observed between CETP mass and VLDL, IDL, or LDL particles. However, CETP mass was positively correlated with the level of small HDL particles in these participants (rs=0.560, p=0.0005).
Analysis by Gender
The key significant correlations between PLTP and lipoproteins in the entire cohort were also examined by gender subgroup. Overall, the results were qualitatively similar between men and women (Table 5). Both men and women revealed positive correlations between PLTP activity or SA and triglyceride, and between PLTP mass and HDL-C, large HDL and mean HDL size. Likewise, the inverse correlation between PLTP SA and HDL-C, large HDL and mean HDL particle size were seen in both genders. These positive or inverse correlations were mostly preserved after controlling for triglyceride in partial correlation analyses. Interestingly, holding triglyceride constant greatly increased the correlations between PLTP activity and HDL-C, large HDL, and mean HDL size in men but not in women.
Table 5.
Bivariate and Partial Correlations between PLTP and Lipoproteins by Gender
| PLTP Activity | PLTP Mass | PLTP SA | ||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| Spearman Correlation Coefficient | ||||||
| Triglyceride | 0.662*** | 0.286 | −0.155 | −0.447* | 0.572** | 0.462* |
| HDL Cholesterol | 0.240 | −0.041 | 0.638*** | 0.248 | −0.487* | −0.152 |
| Large HDL | 0.146 | 0.027 | 0.568* | 0.473* | −0.574* | −0.306 |
| Mean HDL Size | 0.104 | −0.073 | 0.653*** | 0.497* | −0.696*** | −0.386 |
| Partial Correlation Coefficient Controlling for Triglyceride | ||||||
| HDL Cholesterol | 0.501* | 0.039 | 0.601** | 0.176 | −0.442 | −.101 |
| Large HDL | 0.607** | −0.053 | 0.485 | 0.391 | −0.224 | −0.329 |
| Mean HDL Size | 0.518* | −0.120 | 0.580* | 0.461 | −0.437 | −0.405 |
p≤0.05
p≤0.01
p≤0.005 denote significant correlation between PLTP and lipoproteins
Lipoproteins and Variability of Plasma PLTP
As significant correlations were observed between select lipoproteins and PLTP parameters, the extent these lipoproteins contribute to the variation of plasma PLTP was studied using simple and multiple linear regression analysis. In multiple analyses, PLTP activity, mass, or SA were the response variables, and the entities that correlated with each of these variable at p≤0.01 were entered as explanatory variables. Both forward and backward stepwise models were carried out. When plasma BMI, cholesterol, triglyceride, apo B, and large VLDL were entered in the models, triglyceride was the only parameter that contributed significantly to the variation of plasma PLTP activity with an R2 of 0.352. When HDL-C, large HDL, mean LDL size, and mean HDL size were entered in the models, HDL-C best explained the variability of PLTP mass with an R2 of 0.240. When triglyceride (the strongest predictor of PLTP activity), HDL-C (the strongest predictor of PLTP mass), and the lipoprotein particles showing significant correlations with PLTP SA (see Table 4) were entered in the model, HDL particle mean size was the only significant predictor of plasma PLTP SA with an R2 of 0.451. Thus, HDL particle size explained 45% of the variability of plasma PLTP SA. If lipoprotein particles were excluded from the model, both triglyceride and HDL-C were significantly associated with plasma PLTP SA. The standardized regression coefficients for HDL-C and triglyceride were −0.323 (p=0.034) and 0.406 (p=0.009), respectively, and R2 for the model was 0.392.
DISCUSSION
PLTP Activity
In this population of apparently healthy non-smokers not taking any medication or hormones, plasma PLTP activity was positively and significantly associated with BMI, total cholesterol, triglyceride, apo B, and VLDL particles. These bivariate correlations are entirely consistent with the results of several previous studies performed on larger numbers of subjects with different lipid and metabolic characteristics [19–21] although there was one report showing an inverse correlation [22] and another showing no correlation between PLTP activity and some of the above parameters [23]. The observation that controlling for triglyceride reduced the correlation of BMI, cholesterol, apo B, and VLDL particles with PLTP activity to the point that the correlations were no longer significant (Table 2 and Table 4) suggests that their association with PLTP activity was primarily due to their mutual association with triglyceride. Indeed, in multiple linear regression analysis, only triglyceride was significantly related to plasma PLTP activity and 34% of the total variability of plasma PLTP activity could be explained by triglyceride.
PLTP mass
In bivariate analysis, plasma PLTP mass was significantly and positively associated with the amount of HDL-C and large HDL particles and with the mean particle size of HDL and LDL. The association of PLTP mass with HDL-C has been previously reported [24–27]. Here we found remarkable relationships between PLTP mass and lipoprotein particle size. Partial correlation controlling for triglyceride weakened significantly the correlation of PLTP mass with LDL size but not with HDL size or HDL-C. In these and other subjects [18], mean HDL and LDL size were highly correlated (r≥0.7). Thus, the correlation between PLTP mass and the various HDL parameters are independent of triglyceride but the bivariate correlation observed between LDL size and PLTP mass was primarily due to the strong relationship between LDL and HDL size. In linear models, HDL-C was the best predictor of PLTP mass, accounting for 24% of the variability of PLTP in plasma.
Consistent with most previous studies [24–27], no significant bivariate correlation was seen between PLTP mass and activity. However, once adjusted for triglyceride, a significant correlation emerged between PLTP mass and PLTP activity. This effect of triglyceride on the correlation between PLTP mass and PLTP activity was also noted in previous reports [24,27].
PLTP specific activity
Analysis of the relationship between plasma PLTP SA and lipoprotein particles showed several significant bivariate correlations between PLTP SA and selected subclasses of VLDL, LDL, and HDL. PLTP SA was positively correlated with large and medium VLDL, IDL, and small LDL but inversely correlated with large LDL, large HDL, and mean particle size of LDL and HDL. The association between PLTP SA and HDL size was independent of triglyceride but the association of PLTP SA with all non-HDL particles was triglyceride-dependent. Of particular importance to our understanding of the relationship between PLTP and lipoproteins are the observations that (1) the inverse association between plasma PLTP SA and HDL particles was confined to large particles only, suggesting the possibility that PLTP associated with large HDL particles has lower specific activity than PLTP associated with small HDL particles, as small HDL particles appeared to be positively associated with PLTP SA (rs=0.345, p=0.042, Table 4). (2) Mean HDL particle size alone could explain 45% of the total variability of plasma PLTP SA in linear models, and without consideration of lipoprotein particle data, plasma triglyceride and HDL-C together predicted 39% of PLTP SA.
Implications and Significance
Human plasma contains two forms of PLTP [14–16]. The “active” form has the ability to transfer phosphatidylcholine from phospholipid vesicles to HDL and the “inactive” form has little or no ability to transfer phospholipids under similar conditions. Between 55 and 90% of PLTP in normal plasma has been reported to be in the inactive form [15, 28]. The origin, regulation, and significance of these two pools of PLTP are at present unknown. HepG2 conditioned medium appears to contain only the active form of PLTP associated with apo E but not apo A-I [29]. This finding and other studies [15, 30] lead to the hypothesis that nascent PLTP enters the circulation as the active form associated with apo E but not with HDL and that when PLTP attaches to the apo A-I-containing particles during the transfer of surface phospholipids from triglyceride-rich lipoproteins to HDL, it loses its ability to transfer phospholipids. While noting that some work [2] does not agree with this specific concept [15, 30], the present data do imply a role for HDL particle size in the regulation of the pool size of low specific activity PLTP. Our findings that PLTP mass is positively and specifically associated with large HDL particle levels and that the strong independent inverse correlation between PLTP SA and HDL particles is also confined to large HDL particles (but not medium or small HDL particles) imply that the association of PLTP with apo A-I-containing lipoproteins per se does not inactivate PLTP, but rather that binding of PLTP to large HDL particles compromises its ability to transfer phospholipids. This concept is consistent with the reports that the average size of inactive PLTP complexes (520 KDa, 12 to >17nm) are larger than active PLTP complexes (160 KDa, 7.6–12.0 nm) [14, 16]. It is also consistent with an earlier report showing that inactive PLTP is nearly absent in the plasma of individuals with lecithin-cholesterol acyltransferase (LCAT) deficiency, Tangier disease, apo A-I deficiency, or familial HDL deficiency, all of whose HDL have few or no large HDL particles [26]. Large and small HDL particles differ in their lipid, apoA-I and apoA-II composition [31]. These differences likely lead to different protein-protein and protein-lipid interactions between PLTP and HDL and consequently differences in the phospholipid transfer ability among PLTP molecules (i.e. differences in specific activity). This possibility is supported by previous studies showing that the apoA-I, apoA-II, cholesterol and triglyceride contents of HDL, as well as electrostatic interactions between PLTP and lipoproteins and the size of donor-acceptor particles can influence the rate of PLTP-mediated phospholipid transfer and HDL interconversions [32–36]. PLTP can facilitate the transfer of surface lipid from triglyceride-rich lipoproteins or cells to HDL and pre-beta AI. These transfers, coupled with the action of LCAT, result in the conversion of small HDL to large HDL. Based on this concept, we hypothesize that PLTP of low specific activity is generated at the terminus of specific pathways such as lipid transport from triglyceride-rich lipoproteins or cells. Since proteins can transfer among HDL particles and large HDL can be converted to smaller particles through the actions of CETP and hepatic lipase, we further hypothesize that PLTP of low specific activity can regain its lipid transfer activity when large HDL is converted to smaller HDL, thereby contributing to mechanisms for interconversion between PLTP of low and high specific activity. Assuming that the size of these two pools of PLTP is regulated by HDL particles in plasma can provide an explanation for the remarkable finding that the mean HDL particle size contributes to 45% of the variability of plasma PLTP SA. Hence, we speculate that HDL-dependent interconversions of PLTP of low and high specific activity could be a mechanism through which PLTP activity may be regulated under normal physiological conditions. However, it should be emphasized that it is still unclear whether PLTP of low and high specific activity are distinct metabolic pools or opposite ends of a continuous spectrum of PLTP specific activity.
In summary, we have shown that in a population of healthy individuals, triglyceride and HDL-C contribute to 34% and 24% of the total variability of plasma PLTP activity and mass, respectively. Importantly, a strong inverse relationship exists between PLTP SA and large HDL, and HDL particle size could explain 45% of the total variability of plasma PLTP SA. These data and previous studies suggest that HDL particle size plays an important role in regulating the relative amounts of PLTP of low and high specific activity.
ACKNOWLEDGEMENT
This work was supported by NHLBI grants HL030086 (J.J.A.) and HL021544 (J.H.G.).
REFERENCES
- 1.Day JR, Albers JJ, Lofton-Day CE, Gilbert TL, Ching AF, Grant FJ, O’Hara PJ, Marcovina SM, Adolphson JL. Complete cDNA encoding human phospholipid transfer protein from human endothelial cells. J. Biol. Chem. 1994;69:9388–9391. [PubMed] [Google Scholar]
- 2.Cheung MC, Albers JJ. Active plasma phospholipid transfer protein is associated with apoA-I- but not apoE-containing lipoproteins. J. Lipid Res. 2006;47:1315–1321. doi: 10.1194/jlr.M600042-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Tall AR, Krumholz S, Olivecrona T, Deckelbaum RJ. Plasma phospholipid transfer protein enhances transfer and exchange of phospholipids between very low density lipoproteins and high density lipoproteins during lipolysis. J. Lipid Res. 1985;26:842–851. [PubMed] [Google Scholar]
- 4.Tollefson JH, Ravnik S, Albers JJ. Isolation and characterization of a phospholipid transfer protein (LTP-II) from human plasma. J. Lipid Res. 1988;29:1593–1602. [PubMed] [Google Scholar]
- 5.Cheung MC, Wolfbauer G, Albers JJ. Plasma phospholipid mass transfer rate: relationship to plasma phospholipid and cholesteryl ester transfer activities and lipid parameters. Biochim. Biophys. Acta. 1996;1303:103–110. doi: 10.1016/0005-2760(96)00082-3. [DOI] [PubMed] [Google Scholar]
- 6.Nishida HI, Nishida T. Phospholipid transfer protein mediates transfer of not only phosphotidylcholine but also cholesterol from phosphotidylcholine-cholesterol vesicles to high density lipoproteins. J. Biol. Chem. 1997;272:6959–6964. doi: 10.1074/jbc.272.11.6959. [DOI] [PubMed] [Google Scholar]
- 7.Tu A-Y, Nishida HI, Nishida T. High-density lipoprotein conversion mediated by human plasma phospholipid transfer protein. J. Biol. Chem. 1993;268:23098–23105. [PubMed] [Google Scholar]
- 8.Albers JJ, Wolfbauer G, Cheung MC, Day JR, Ching AF, Lok S, Tu A-Y. Functional expression of human and mouse plasma phospholipid transfer protein: effect of recombinant and plasma PLTP on HDL subspecies. Biochim. Biophys. Acta. 1995;1258:27–34. doi: 10.1016/0005-2760(95)00091-p. [DOI] [PubMed] [Google Scholar]
- 9.von Eckardstein A, Jauhiainen M, Huang Y, Metso J, Langer C, Pussinen P, Wu S, Ehnholm C, Assmann G. Phospholipid transfer protein mediated conversion of high density lipoproteins generates prebeta-HDL. Biochim. Biophys. Acta. 1996;1301:255–262. doi: 10.1016/0005-2760(96)00050-1. [DOI] [PubMed] [Google Scholar]
- 10.Wolfbauer G, Albers JJ, Oram JF. Phospholipid transfer protein enhances removal of cellular cholesterol and phospholipids by high-density lipoprotein apolipoproteins. Biochim. Biophys. Acta. 1999;1439:65–76. doi: 10.1016/s1388-1981(99)00077-3. [DOI] [PubMed] [Google Scholar]
- 11.Oram JF, Wolfbauer G, Vaughan AM, Tang C, Albers JJ. Phospholipid transfer protein interacts with and stabilizes ATP-binding cassette transporter A1 and enhances cholesterol efflux from cells. J. Biol. Chem. 2003;278:52379–52385. doi: 10.1074/jbc.M310695200. [DOI] [PubMed] [Google Scholar]
- 12.Qin S, Kawano K, Bruce C, Lin M, Bisgaier C, Tall AR, Jiang X. Phospholipid transfer protein gene knock-out mice have low high density lipoprotein levels, due to hypercatabolism, and accumulate apoA-IV-rich lamellar lipoproteins. J. Lipid Res. 2000;41:269–276. [PubMed] [Google Scholar]
- 13.Van Tol A, Jauhiainen M, De Crom R, Ehnholm C. Role of phospholipid transfer protein in high-density lipoprotein metabolism: insights from studies in transgenic mice. Int. J. Tissue React. 2000;22:79–84. [PubMed] [Google Scholar]
- 14.Oka T, Kujiraoka T, Ito M, Egashira T, Takahashi S, Nanjee MN, Miller NE, Metso J, Olkkonen VM, Ehnholm C, Jauhianinen M, Hattori H. Distribution of phospholipid transfer protein in human plasma: presence of two forms of phospholipid transfer protein, one catalytically active and the other inactive. J. Lipid Res. 2000;41:1651–1657. [PubMed] [Google Scholar]
- 15.Karkkainen M, Oka T, Olkkonen VM, Metso J, Hattori H, Jauhiainen M, Ehnholm C. Isolation and partial characterization of the inactive and active forms of human plasma phospholipid transfer protein (PLTP) J. Biol. Chem. 2002;277:15413–15418. doi: 10.1074/jbc.M112247200. [DOI] [PubMed] [Google Scholar]
- 16.Murdoch SJ, Wolfbauer G, Kennedy H, Marcovina SM, Carr MC, Albers JJ. Differences in reactivity of antibodies to active versus inactive PLTP significantly impact PLTP measurement. J. Lipid Res. 2002;43:281–289. [PubMed] [Google Scholar]
- 17.Deguchi H, Fernandez JA, Griffin JH. Plasma cholesteryl ester transfer protein and blood coagulability. Thromb. Haemost. 2007;98:1160–1164. [PubMed] [Google Scholar]
- 18.Jeyarajah EJ, Cromwell WC, Otvos EJ. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Tahvanainen E, Jauhiainen M, Funke H, Vartiainen E, Sundvall J, Ehnholm C. Serum phospholipid transfer protein activity and genetic variation of the PLTP gene. Atherosclerosis. 1999;146:107–115. doi: 10.1016/s0021-9150(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 20.Cheung MC, Knopp RH, Retzlaff B, Kennedy H, Wolfbauer G, Albers JJ. Association of plasma phospholipid transfer protein activity with IDL and buoyant LDL: impact of gender and adiposity. Biochim. Biophys. Acta. 2002;1587:53–59. doi: 10.1016/s0925-4439(02)00054-6. [DOI] [PubMed] [Google Scholar]
- 21.Colhoun HM, Taskinen MR, Otvos JD, Van Den Berg P, O'Connor J, Van Tol A. Relationship of phospholipid transfer protein activity to HDL and apolipoprotein B-containing lipoproteins in subjects with and without type1 diabetes. Diabetes. 2002;51:3300–3305. doi: 10.2337/diabetes.51.11.3300. [DOI] [PubMed] [Google Scholar]
- 22.Ooi EMM, Watts GF, Ji J, Rye K-A, Johnson AG, Chan DC, Barrett PHR. Plasma phospholipid transfer protein activity, a determinant of HDL kinetics in vivo. Clin. Endocrin. 2006;65:752–759. doi: 10.1111/j.1365-2265.2006.02662.x. [DOI] [PubMed] [Google Scholar]
- 23.Desrumaux C, Athias A, Bessede G, Verges B, Farnier M, Persegol L, Gambert P, Lagrost L. Mass concentration of plasma phospholipid transfer protein in normolipidemic, type IIa hyperlipidemic, type IIb hyperlipidemic, and non-insulin-dependent diabetic subjects as measured by a specific ELISA. Arterioscler. Thromb. Vasc. Biol. 1999;19:266–275. doi: 10.1161/01.atv.19.2.266. [DOI] [PubMed] [Google Scholar]
- 24.Huuskonen J, Ekstrom M, Tahvanainen E, Vainio A, Metso J, Pussinen P, Ehnholm C, Olkkonen VM, Jauhiainen M. Quantification of human plasma phospholipid transfer protein (PLTP): relationship between PLTP mass and phospholipid transfer activity. Atherosclerosis. 2000;151:451–461. doi: 10.1016/s0021-9150(99)00429-3. [DOI] [PubMed] [Google Scholar]
- 25.Oka T, Kujiraoka T, Ito M, Nagano M, Ishihara M, Iwasaki T, Egashira T, Miller NE, Hattori H. Measurement of human plasma phospholipid transfer protein by sandwich ELISA. Clin. Chem. 2000;46:1357–1364. [PubMed] [Google Scholar]
- 26.Oka T, Yamashita S, Kujiraoka T, Ito M, Nagano M, Sagehashi Y, Egashira T, Nanjee MN, Hirano K-I, Miller NE, Matsuzawa Y, Hattori H. Distribution of human plasma PLTP mass and activity in hypo- and hyperalphalipoprotienemia. J. Lipid Res. 2002;43:1236–1243. [PubMed] [Google Scholar]
- 27.Dullaart RP, De Vries R, Scheek L, Borggreve SE, Van Gent T, Dallinga-Thie GM, Ito M, Nagano M, Sluiter WJ, Hattori H, Van Tol A. Type 2 diabetes mellitus is associated with differential effects on plasma cholesteryl ester transfer protein and phospholipid transfer protein activities and concentrations. Scand. J. Clin. Lab. Invest. 2004;64:205–215. doi: 10.1080/00365510410005721. [DOI] [PubMed] [Google Scholar]
- 28.Jänis MT, Siggins S, Tahvanainen E, Vikstedt R, Silander K, Metso J, Aromaa A, Taskinen MR, Olkkonen VM, Jauhiainen M, Ehnholm C. Active and low-active forms of serum phospholipid transfer protein in a normal Finnish population sample. J. Lipid Res. 2004;45:2303–2309. doi: 10.1194/jlr.M400250-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Siggins S, Jauhiainen M, Olkkonen VM, Tenhunen J, Ehnholm C. PLTP secreted by HepG2 cells resembles the high-activity PLTP form in human plasma. J. Lipid Res. 2003;44:1698–1704. doi: 10.1194/jlr.M300059-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Janis MT, Metso J, Lankinen H, Strandin T, Olkkonen VM, Rye K-A, Jauhiainen M, Ehnholm C. Apolipoprotein E activates the low-activity form of human phospholipid lipid transfer protein. Biochem. Biophys. Res. Commun. 2005;331:333–340. doi: 10.1016/j.bbrc.2005.03.164. [DOI] [PubMed] [Google Scholar]
- 31.Cheung MC, Nichols AV, Blanche PJ, Gong EL, Franceschini G, Sirtori CR. Characterization of A-I-containing lipoproteins in subjects with A-I Milano variant. Biochim. Biophys. Acta. 1988;960:73–82. doi: 10.1016/0005-2760(88)90011-2. [DOI] [PubMed] [Google Scholar]
- 32.Huuskonen J, Olkkonen VM, Jauhiainen M, Metso J, Somerharju P, Ehnholm C. Acyl chain and headgroup specificity of human plasma phospholipid transfer protein. Biochim. Biophys. Acta. 1996;1303:207–214. doi: 10.1016/0005-2760(96)00103-8. [DOI] [PubMed] [Google Scholar]
- 33.Pussinen PJ, Jauhiainen M, Ehnholm C. ApoA-II/apoA-I molar ratio in the HDL particle influences phospholipid transfer protein-mediated HDL interconversion. J. Lipid Res. 1997;38:12–21. [PubMed] [Google Scholar]
- 34.Nishida HI, Nishida T. Phospholipid transfer protein mediates transfer of not only phosphatidylcholine but also cholesterol from phosphatidylcholine-cholesterol vesicles to high density lipoproteins. J. Biol. Chem. 1997;272:6959–6964. doi: 10.1074/jbc.272.11.6959. [DOI] [PubMed] [Google Scholar]
- 35.Rao R, Albers JJ, Wolfbauer G, Pownall HJ. Molecular and macromolecular specificity of human plasma phospholipid transfer protein. Biochemistry. 1997;36:3645–3653. doi: 10.1021/bi962776b. [DOI] [PubMed] [Google Scholar]
- 36.Settasatian N, Duong M, Curtiss LK, Ehnholm C, Jauhiainen M, Huuskonen J, Rye K-A. The mechanism of the remodeling of high density lipoproteins by phospholipid transfer protein. J. Biol. Chem. 2001;276:26898–26905. doi: 10.1074/jbc.M010708200. [DOI] [PubMed] [Google Scholar]