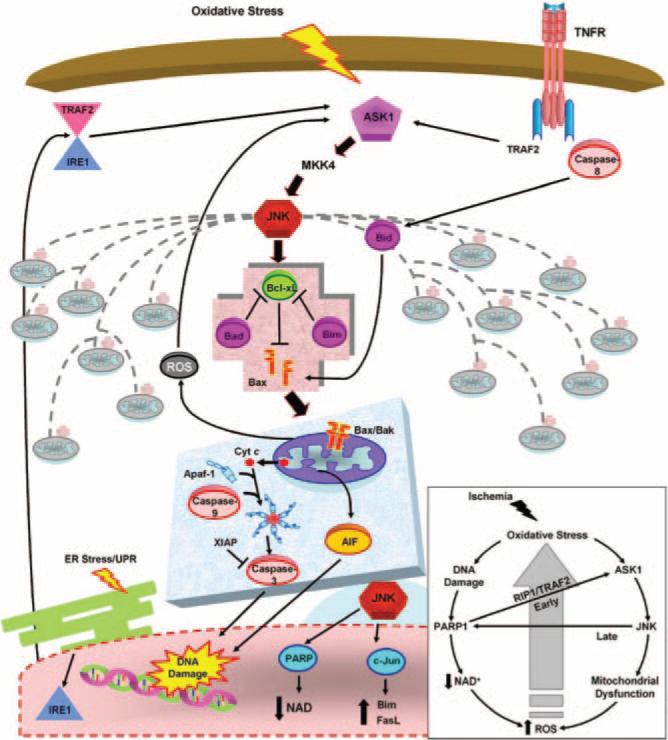
Figure 1.
Targeting the ASK1/MKK4/JNK pathway upstream of mitochondrial prodeath signaling. Oxidative stress caused by cerebral ischemia results in activation of mitochondrial death signaling. Initially activated by oxidative stress, ASK1 signals JNK activation via MKK4. Activated JNK triggers prodeath Bcl-2 proteins to disrupt the mitochondrial membrane. Subsequent release of downstream mitochondrial death effectors ensues, causing DNA damage and ultimate neuronal death. Alternative stresses that act at later times after ischemia, such as ER stress and inflammation, can also activate ASK1/MKK4/JNK signaling. In addition to the direct signaling consequences, JNK activation results in a genomic response, altering the expression of key regulators of neuronal death. Inset: Oxidative stress simultaneously causes DNA damage and ASK1 activation. DNA damage activates PARP1, leading to NAD depletion and increased ROS. ASK1 results in mitochondrial damage and increased ROS. The increase in ROS causes further oxidative stress and propagation of the cycle. Cross-talk also occurs as PARP1 activation can activate ASK1, and JNK activation can also activate PARP at later times after ischemia. Progressive ROS accumulation causes increased JNK activation and damage to multiple mitochondria, ensuring neuronal demise. AIF indicates apoptosis-inducing factor; Apaf-1, apoptosis-activating factor-1; ASK1, apoptosis signal-regulating kinase 1; cyt c, cytochrome c; ER, endoplasmic reticulum; FasL, Fas ligand; IRE1, inositol requirement 1; JNK, c-Jun–terminal kinase; NAD, nicotinamide adenine dinucleotide; PARP, poly (ADP-ribose) polymerase; RIP1, receptor interacting protein 1; ROS, reactive oxygen species; TRAF2, TNF receptor-associated factor 2; TNFR, tumor necrosis factor receptor; XIAP, X-linked inhibitor of apoptosis.