Abstract
To determine if the involuntary contractions of eyelids may have any effects on the development of corneal astigmatism, we performed this prospective study which includes 19 patients with either essential blepharospasm or hemifacial spasm. In hemifacial spasm, the degree of corneal astigmatism was evaluated between two eyes. Then the topographic changes were checked using vector analysis technique before and after passively opening the eyelids. They were also measured before and at 1 and 6 months after the injection of Botulinum toxin. Resultantly, 20 eyes had the with-the-rule (group1) and 9 eyes against-the-rule (group2) astigmatism. In hemifacial spasm, significantly more astigmatism was found at spastic eyes. The corneal topographic changes after passively opening the eyelids showed 10 eyes with the astigmatic shift to the with-the-rule, while the remaining 19 to the against-the-rule. At 1 month after injection of Botulinum toxin, group 1 showed reduced average corneal astigmatism, whereas group 2 showed increased astigmatism. The astigmatic change vector showed significantly more against-the-rule. In the contrary, 6 months after treatment, corneal astigmatism again increased in group 1 and decreased in group 2. So they took on the appearance of pretreatment astigmatic status eventually. Conclusively eyelids may play an important role in corneal curvature.
Keywords: Astigmatism, Blepharospasm, Botulinum Toxin Type A, Corneal Topography, Eyelids
INTRODUCTION
Astigmatism is one of the most important causes of ocular fatigue and visual disturbance. It is primarily caused by the optical center not being focused at one point due to the differences of refractive power from each meridian of the anterior, and posterior corneal surfaces and the lens itself. Among them the corneal astigmatism accounts for most of the ocular astigmatism.
In fact, the cause of corneal astigmatism remains still unclear. Since Snellen first postulated that eyelids might affect the corneal shape and astigmatism in the 19th century, many other studies have reported reciprocal results. Among them, Gullstrand suggested that if the cornea were not influenced by the external pressure, it would maintain against-the-rule astigmatism and that with-the-rule astigmatism would occur due to the lid tension imposed on the corneal surface (1). Similarly, we can often see with-the-rule astigmatism in those with high lid pressure like children, whereas against-the-rule astigmatism develops with the reduction of lid pressure in the aged. The evidence that the pressure of eyelids has a direct effect on corneal shape can be found in common clinical situations (2). The chalazia in upper eyelid have been shown to produce a reduction in vision, associated with reversible central corneal flattening (3, 4). In the interpretation of the normal corneal topographies, we have to take some other factors into considerations. First, alteration in the distribution of tear can induce the corneal topographic changes (5). It is because the topographic images are taken from the most anterior ocular surface, and the physiologic outermost ocular surface is made up of tear film. Its conformation can change over time after blinking and the tear layer is known to reach its most regular state at 3-10 sec after each blink (6). We also have to consider diurnal variations of corneal curvature and other systemic factors such as age, gender, and intraocular pressure to evaluate the change of corneal topography (7, 8).
In this study, we tried to investigate the role of eyelids in the development of corneal astigmatism in patients who have involuntary lid spasm, by observing that the induced astigmatism could be released with the removal of the mechanical pressure of the lid using Botulinum toxin-A. In the course of spasm, the tension of superior lid was so strong that we could ignore the effect of other subtle confounding factors.
MATERIALS AND METHODS
With the informed consent, we studied 19 patients with 27 eyes who suffered from essential blepharospasm or hemifacial spasm involving lids for more than 1 yr and visited Yongsan Hospital, Chung-Ang University from February 2001 to January 2003. After noting age and sex, the corneal astigmatism was measured using a videokeratography (Keratograph®, Oculus, Wetzlar, Germany). The lid tension was measured subjectively by grasping eyelashes with two fingers and pulling them outward and upward. We excluded from our study the patients in whom the corneal astigmatism could not be measured by this method due to their severe blepharospasm and the patients with other ocular problems that could cause any corneal surface alterations.
To evaluate the direct mechanical effects of eyelids, we evaluated the keratometric changes in 3 ways. At first, we compared the differences of astigmatism between two eyes of a patient having hemifacial spasm without any external forces exerted on the eyelids. Then the topographic changes were also measured before and after passively opening the eyelids in all the patients enrolled in our study, paying attention not to dry the cornea. It was taken at 5 to 10 sec after their eyes were passively opened by the speculum. And at the same time, special efforts were made to avoid external forces evoked by hands or speculum exerting on lids and to avoid meibomian secretion running down into the tear layer. In this manner, we could minimize the effects of tear flow, and thus we could evaluate the short-term influence of eyelids on corneal topography. And to evaluate the long term changes of corneal astigmatism after removal of mechanical effects of eyelids, we used botulinum toxin-A (Botox®, Allergan, Irvine, CA, U.S.A.). It was stored at -20℃ and diluted with saline solution just before injection. A dose of 4-unit/0.1 mL was injected into the orbicularis oculi, corrugator superficilii and procerus muscles with a 30-gauge needle respectively. All the procedures were conducted by the same physician. The follow-up examinations for the relaxation of spasm and resultant changes of corneal astigmatism were performed with their eyes open spontaneously at 1 and 6 months after the injection, because the effect of toxin was to reach its maximum by 1 month and disappear by 6 months after injection into musculature (9).
We analyzed the amount and steepest axis of central corneal astigmatism from the keratometric values obtained from videokeratoscopy. We also defined with-the-rule astigmatism when its steepest meridian lies from 45° to 135° and against-the-rule astigmatism from 0° to 45° and 135° to 180°. In order to evaluate the changes of astigmatism after treatment, double-angled vector analysis technique was used, as the axis of astigmatic vector has the same value by rotating 180° arc (10, 11). We set each circle's interval at 0.5 diopter (D). In this map, the right side of circle revealed against-the-rule astigmatism and left side revealed with-the-rule astigmatism. Corneal astigmatism change vector was calculated using the following equation below, and then the change of vector point was marked in the double angled map.
ΔV=Vector after (botulinum toxin-A) injection-Vector before injection
If the vector points of astigmatic change after botulinum toxin injection lay in right side of the map, we could tell that they had the tendency towards against-the-rule astigmatism and accordingly the left side towards with-the-rule astigmatism (Fig. 1).
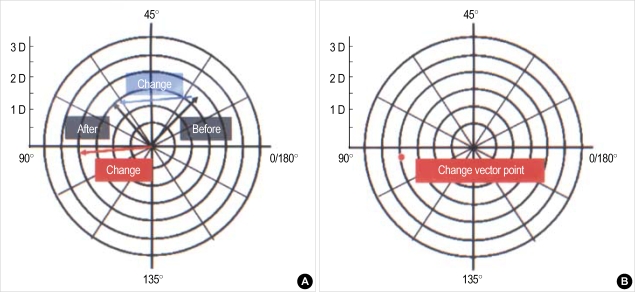
Fig. 1.
Graphical method for calculating corneal astigmatism vector change. (A) If a corneal astigmatic measurement of a patient before botulinum toxin-A injection showed 1.75 diopter with its steepest axis 26 degree (right, marked as "Before") and changed to 1.5 diopter with 62 degree after treatment (left, marked as "After") then the resultant change vector is obtained by subtracting the preoperative value from the postoperative value (blue line). If the vector is drawn to the center (red line) then the apex reveals astigmatic change vector point (B). This case shows with-the-rule change (1 interval: 0.5 D).
The refractive data were measured three times at each visit. The measurements were performed before treatment, at 1 month after injection and 6 months after injection. According to the pretreatment astigmatic values, we divided the patients into two groups as with-the-rule (group 1), and against-the-rule (group 2), and we compared the mean corneal astigmatism between two groups.
All our data in this study were analyzed with 95% confidence interval (Student t-test. SPSS ver. 10.0)
RESULTS
Corneal measurements taken before botulinum toxin-A injection revealed that the patients having with-the-rule astigmatism outnumbered those having against-the-rule astigmatism. Of 19 patients, 8 were male and 11 were female. Of the 29 eyes enrolled in this study, 20 eyes showed with-the-rule astigmatism (group 1) and 9 eyes against-the-rule astigmatism (group 2). Mean age was 62.6±12.9 yr and 13 patients have hemifacial spasm including eyelids and 8 patients have essential blepharospasm.
The astigmatic differences between two eyes in patients with hemifacial spasm
Among the 13 patients with hemifacial spasm, most of them had with-the-rule astigmatism and the involved eyes showed significantly more astigmatism than the opposite healthy eyes (p<0.05, t-test) (Fig. 2A).
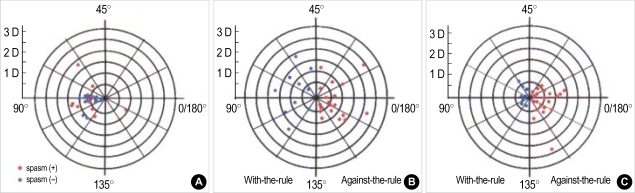
Fig. 2.
(A) The difference of astigmatism between two eyes of a patient having hemifacial spasm. Blue circle is representative for the amount and axis of astigmatism of healthy eye, while red circle for those of spastic eyes. Most of them have the against-the-rule astigmatism. The mean value is 0.85±0.24 D for the involved eyes and 0.58±0.18 D for the healthy eye. It was significantly different. (p<0.05 by t-test) (B) The corneal topographic changes after passively opening the eyelids in spastic eyes. 10 out of 29 eyes show the rapid astigmatic changes to the with-the-rule, while the remaining 19 to the against-the-rule astigmatism. (C) Corneal astigmatism change vector 1 month after botulinum toxin-A injection in patients with blepharospasm. In 21 eyes, astigmatic patterns change to against-the rule (right side, red) and in 8 eyes, to with-the-rule (left side, blue). Distance from center represents diopteric change.
The short-term influence of eyelids on the corneal astigmatism
The swift corneal topographic changes after passively opening the eyelids showed various results, and they were shown as a vector plot (Fig. 2B). Ten eyes showed the astigmatic changes to the with-the-rule, while the most remaining 19 eyes to the against-the-rule.
The long-term influence of eyelids on the corneal astigmatism after injection of Botulinum toxin-A
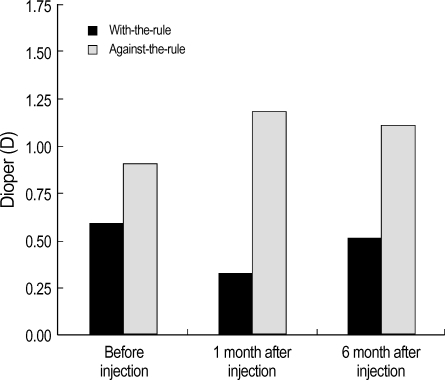
The average values of corneal astigmatism measured before botulinum toxin-A injection, and 1 month and 6 months after injection were 0.59±0.18 D, 0.32±0.15 D and 0.51±0.21 D in group 1, and 0.91±0.23 D, 1.18±0.29 D and 1.11±0.24 D in group 2 respectively. Regarding the changes of corneal astigmatism, the mean value significantly decreased in all the patients of group 1, but the mean value significantly increased although some of them did not show any remarkable changes in group 2 (Table 1, p<0.05, Student t-test).
Table 1.
Mean corneal astigmatism after injection of botulinum toxin-A in patients with blepharospasm (mean±standard deviation)
*p<0.05 by student t-test.
These showed that the value of with-the-rule astigmatism decreased while the value of against-the-rule astigmatism increased after treatment. Accordingly 1 month after treatment, corneal astigmatic change vector plot showed the shift toward against-the-rule astigmatism (right side of the map) in 21 eyes, and the shift toward with-the-rule astigmatism (left side of the map) in 8 eyes (Fig. 2C). These results revealed that most of the patients experienced change of astigmatism toward the against-the-rule astigmatism at about 1 month post-injection (Fig. 3).
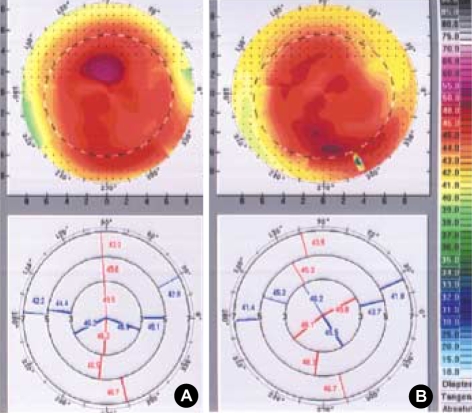
Fig. 3.
The change of corneal topography 1 month after botulinum toxin-A injection. An example of topographic change before (A) and 1 month after injection of botulinum toxin-A (B). This topographic pattern reveals with-the-rule astigmatism before treatment, but slowly changes into against-the-rule astigmatism after toxin injection.
In the contrary, at 6 month after treatment when the effect of botulinum toxin is thought to be nullified, corneal astigmatism again increased in group 1 and decreased in group 2. So they took on the appearance of corneal astigmatism of pretreatment status eventually (Fig. 4).
Fig. 4.
The mean corneal astigmatism before and 1, 6 months after injection of botulinum toxin-A in patients with blepharospasm. With-the-rule astigmatism decreases and against-the rule astigmatism increases at 1 month, but return to its original pattern 6 months after injection of toxin.
DISCUSSION
The causes of corneal astigmatism are not completely understood. Nevertheless, several factors have been found to contribute to the development of corneal astigmatism, and among them the pressure exerted by eyelids has been considered to be one of the most important factors of all. Suat and Guler reported asymmetrically increased corneal astigmatism in patients with congenital ptosis in a single eye, as compared to the healthy eye (12). A recent report also explored a change of corneal astigmatism after implantation of a metallic device in the eyelid for the management of lagophthalmos (13). We can deduce from the above studies that eyelids may place some effects on corneal astigmatism. However, their results were nothing but a short-term effect of removing the lid tension with speculum, which turned out to be highly variable in our study. In fact, for the investigation about the cause of astigmatism, we need to evaluate the relatively long term effect of eyelids, because an external force exerted for a long period can cause some conformational changes on the cornea.
Our results showed that the corneal topography might be influenced by the mechanical pressure of lids by observing the differences of astigmatism between two eyes in hemifacial spastic patients. And we could also see that the astigmatism induced by contraction of lids rapidly changed with the removal of such causative pressure by passively opening them. However, the direction of the changes was not constant in vector analysis (Fig. 2B).
Botulinum is a kind of neurotoxin, which is elaborated by Clostridium botulinum. Its site of action is presynaptic nerve terminal where it prevents the release of acetylcholine. There are diverse types of botulinum toxin in nature, and among them, type-A toxin is the most stable and commercially used for medical purposes (14). Its effects usually last for several months, so after injection of toxin, we can see the relatively long term changes of corneal topography exerted by eyelids.
We observed the reduction of the with-the-rule astigmatism and the increase of the against-the-rule astigmatism at 1 month after botulinum toxin-A injection. But 6 months later, a slow increase in lid tension and a return of corneal astigmatism to the pre-injection value were found. The vector plot revealed the incidences of the changes to the against-the-rule astigmatism were much more than the change to the with-the-rule astigmatism after botulinum toxin-A injection. These were because the patients who suffered from blepharospasm with strong lid tension showed a tendency toward flattening on the periphery of the cornea in superior vertical meridian, and conversely steepening in the corneal center. As a result, what we could see was the formation of the with-the-rule astigmatism in patients with blepharospasm due to the constant pressure of lids. However botulinum toxin-A injection relieved the lid tension, and so it reduced the with-the-rule astigmatism and induced vectorial changes to the against-the-rule astigmatism (Fig. 2C).
Actually, many factors can influence on the change of corneal topography other than the pressure of eyelids. The tear flow after blinking can bring about striking changes in corneal topography, but it usually is temporal and stabilized after several seconds (5, 6). The excessive lipid secretions from the meibomian glands can also form an unstable corneal surface, so we should take care not to squeeze the lid itself during the ocular examination.
It is well known that circadian rhythm has been demonstrated in various parameters of the eyes. Intraocular pressure is measured high in the light awake periods and corneal curvature can be changed accordingly (15). The corneal curvature itself also has diurnal variations, and it is also known to be influenced by the sexual hormone (7, 8). But in comparison with the effect of lid pressure, these changes are considered to be somewhat trivial, so we could disregard them in our study. However, taking all factors into considerations, we tried to check the patient as regularly as possible at each visit. We measured corneal topography at a few seconds after opening the lids by speculum, and we also excluded any patients from our study who showed ocular hypertension.
From our results, we can carefully say that lid pressure can directly exert some influences on corneal topography. But further study is needed to clarify how much tension is needed to change the fixed amount of corneal topography. To do this, it is necessary to measure the lid tension objectively and many kinds of lid tensiometer are now under development and some are already used in some facilities (16).
The consideration of the influence of lid tension is important in that it may be one of the causes in the development of astigmatism in normal population and this may be the reason why we can see more with-the-rule astigmatism in normal population especially in East Asians whose lids are relatively tense. But with a normal palpebral aperture and the eye in the primary gaze, the location of upper lid is usually outside the visual axis, at least more than 3-4 mm from the pupillary center. So it does not affect much the visual function. However, in the condition that can alter the lid posture, like tenacious reading, it can really become bothersome.
Recently the use of botulinum toxin is increasing due to the solely cosmetic purposes and in almost all the ophthalmologic operations and examinations, we passively open the eyelid with either a speculum or fingers. So the corneal shape can be changed and may become different from normal situation resultantly. In evaluating individual refractive status at clinics or in vision improving procedures like photorefractive keratectomy and laser in situ keratomileusis, we should consider the effects of eyelids and inquire if they use botulinum toxin. Otherwise we can encounter the unexpected refractive errors related complications.
References
- 1.Stark L. Presbyopia in light of accommodation. Am J Optom Physiol Opt. 1988;65:407–416. doi: 10.1097/00006324-198805000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Kang KH, Baek SH, Lee KS. Corneal astigmatic change in corneal topography after upper eyelid surgery. J Korean Ophthalmol Soc. 2002;43:1113–1122. [Google Scholar]
- 3.Cosar CB, Rapuano CJ, Cohen EJ, Laibson PR. Chalazion as a cause of decreased vision after LASIK. Cornea. 2001;20:890–892. doi: 10.1097/00003226-200111000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Santa Cruz CS, Culotta T, Cohen EJ, Rapuano CJ. Chalazion-induced hyperopia as a cause of decreased vision. Ophthalmic Surg Lasers. 1997;28:683–684. [PubMed] [Google Scholar]
- 5.Novak KD, Kohnen T, Chang-Godinich A, Soper BA, Kennedy P, Wang Q, Padrick T, Koch DD. Changes in computerized videokeratography induced by artificial tears. J Cataract Refract Surg. 1997;23:1023–1028. doi: 10.1016/s0886-3350(97)80075-2. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth J, Erdelyi B, Csakany B, Gaspar P, Soumelidis A, Kahlesz F, Lang Z. High-speed videotopographic measurement of tear film build-up time. Invest Ophthalmol Vis Sci. 2002;43:1783–1790. [PubMed] [Google Scholar]
- 7.Handa T, Mukuno K, Niida T, Uozato H, Tanaka S, Shimizu K. Diurnal variation of human corneal curvature in young adults. J Refract Surg. 2002;18:58–62. doi: 10.3928/1081-597X-20020101-09. [DOI] [PubMed] [Google Scholar]
- 8.Goto T, Klyce SD, Zheng X, Maeda N, Kuroda T, Ide C. Gender- and age-related differences in corneal topography. Cornea. 2001;20:270–276. doi: 10.1097/00003226-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Calace P, Cortese G, Piscopo R, Della Volpe G, Gagliardi V, Magli A, De Berardinis T. Treatment of blepharospasm with botulinum neurotoxin type A: long-term results. Eur J Ophthalmol. 2003;13:331–336. doi: 10.1177/112067210301300401. [DOI] [PubMed] [Google Scholar]
- 10.Gross RH, Miller KM. Corneal astigmatism after phacoemulsification and lens implantation through unsutured scleral and corneal tunnel incisions. Am J Ophthalmol. 1996;121:57–64. doi: 10.1016/s0002-9394(14)70534-3. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe NS, Jaffe MS, Jaffe GF. Cataract surgery and its complications. St Louis: CV Mosby; 1990. pp. 114–119. [Google Scholar]
- 12.Suat HU, Guler Z. Corneal topography in patients with congenital ptosis. Eye. 1999;13:550–554. doi: 10.1038/eye.1999.136. [DOI] [PubMed] [Google Scholar]
- 13.Lavy JA, East CA, Bamber A, Andrews PJ. Gold weight implants in the management of lagophthalmos in facial palsy. Clin Otolaryngol Allied Sci. 2004;29:279–283. doi: 10.1111/j.1365-2273.2004.00817.x. [DOI] [PubMed] [Google Scholar]
- 14.Waller RR, Kennedy RH, Henderson JW, Kesty KR. Management of blepharospasm. Trans Am Ophthalmol Soc. 1985;83:367–386. [PMC free article] [PubMed] [Google Scholar]
- 15.Smith J. Diurnal intraocular pressure: Correlation to automated perimetry. Ophthalmology. 1985;92:858–861. doi: 10.1016/s0161-6420(85)33926-x. [DOI] [PubMed] [Google Scholar]
- 16.Ehrmann K, Francis I, Stapleton F. A novel instrument to quantify the tension of upper and lower eyelids. Cont Lens Anterior Eye. 2001;24:65–72. doi: 10.1016/s1367-0484(01)80015-1. [DOI] [PubMed] [Google Scholar]