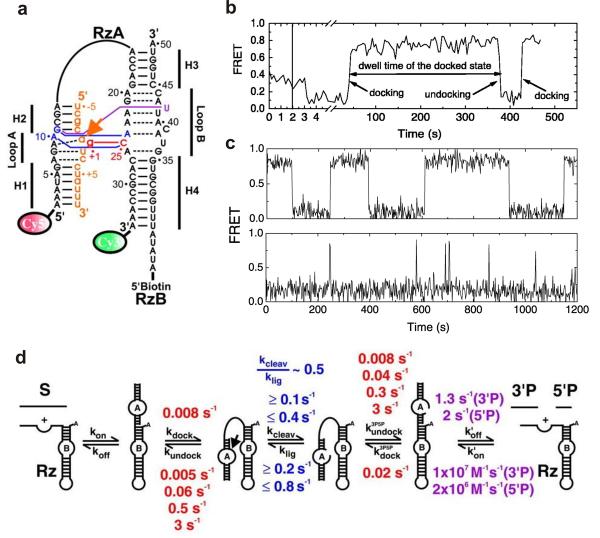
Fig. 1.
Single molecule FRET studies of the hairpin ribozyme. (a) The fluorophore-labeled two-way junction hairpin ribozyme binds with the substrate (orange). Terminal Cy3 and Cy5 fluorophores serve as the fluorescent donor-acceptor pair and biotin is used for surface immobilization through a biotin-streptavidin bridge. (b) A typical sm-FRET time trajectory of a single hairpin ribozyme. The FRET states at 0.8 and 0.2 represent the docked and the undocked states, respectively. (c) The FRET time traces of two different ribozyme molecules exhibit heterogeneous undocking kinetics and reveal the memory effect of the hairpin ribozyme. (d) The multistep reaction pathway of the hairpin ribozyme with the observed rate constants. From reference [33], X. Zhuang et. al., Science 296, 1473 (2002). Reprinted with permission from AAAS.