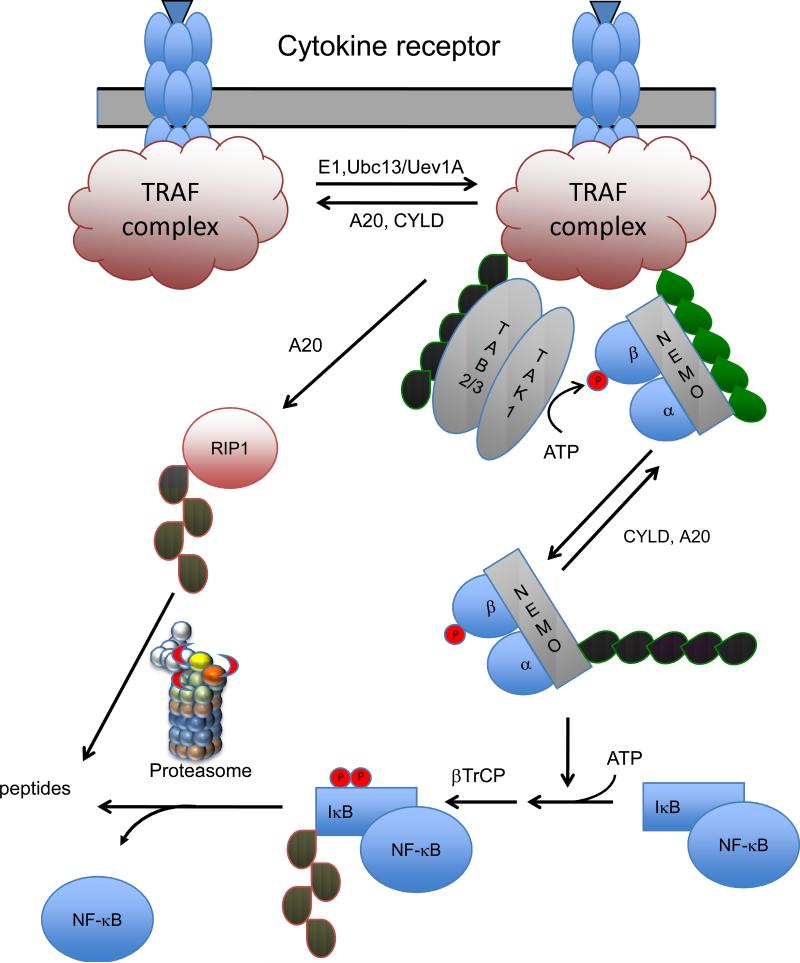
Figure 5.
A generalized model for signaling through the NF-κB pathway. Upon ligand binding to receptors for Tumor Necrosis Factor, Interleukins, Toll ligand, or T- and B-cell antigens a signaling complex is assembled. This complex contains TRAFs (TNF receptor associated factors) that catalyze the formation of a K63-linked polyubiquitin chain (green) on themselves and/or other proteins in the complex. The K63-linked polyubiquitin then recruits the TAB2/3-TAK1 kinase and its substrate IκB kinase (consisting of kinases IKKα, IKKβ, and the regulatory subunit NEMO). Subsequently the phosphorylation of IKKβ and the K63 polyubiquitination of NEMO activates the IKK activity. IKK phosphorylates IκB triggering its K48 polyubiquitination (red) by βTRCP and its degradation by the proteasome. The liberated NF-κB then enters the nucleus and acts as a transcription factor activating genes involved in inflammation and immune responses. The signaling is downregulated by two K63 specific DUBs, A20 and CYLD and defects in either lead to prolonged signaling. RIP1, a component of the TRAF complex assembled upon TNFR1 signaling is one of the targets for K63 polyubiquitination. K63 polyubiquitinated RIP1 is deubiquitinated by A20. This DUB is also a ubiquitin ligase that then assembles a K48 polyubiquitin chain on RIP1 leading to its degradation by the proteasome.