Abstract
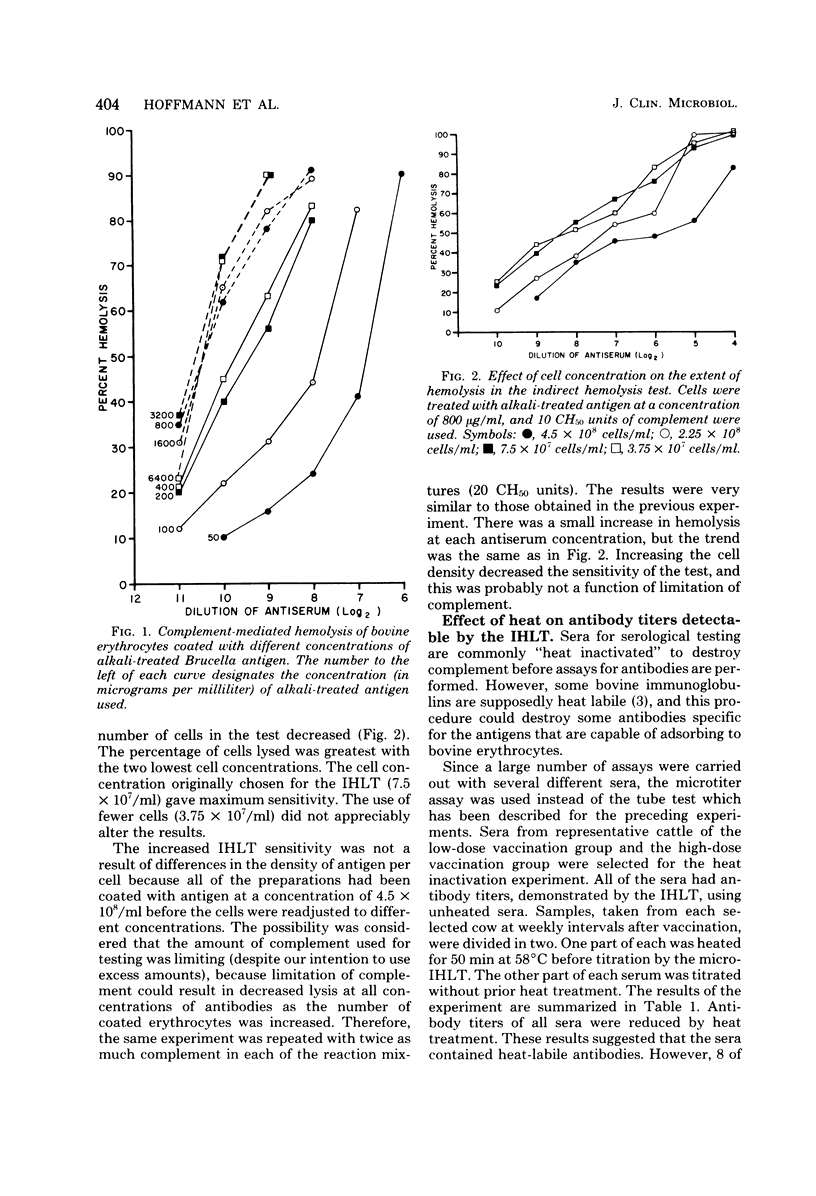
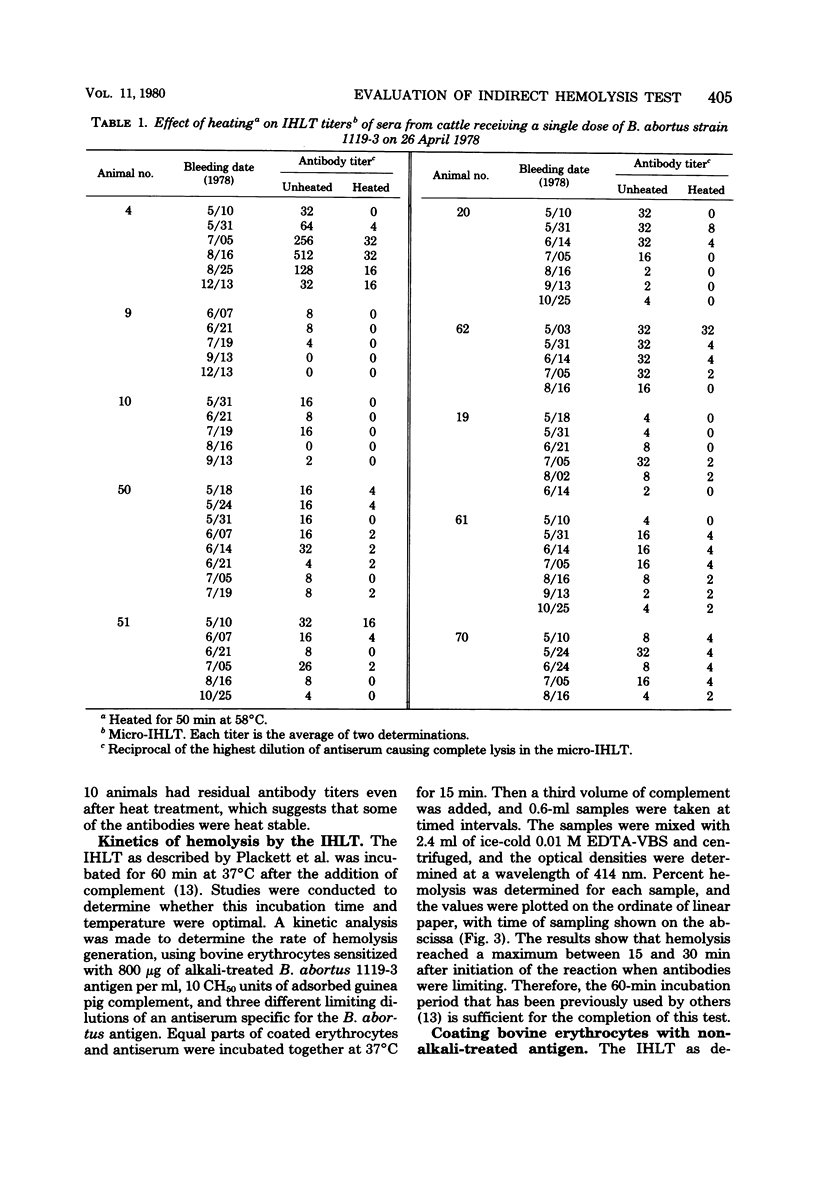
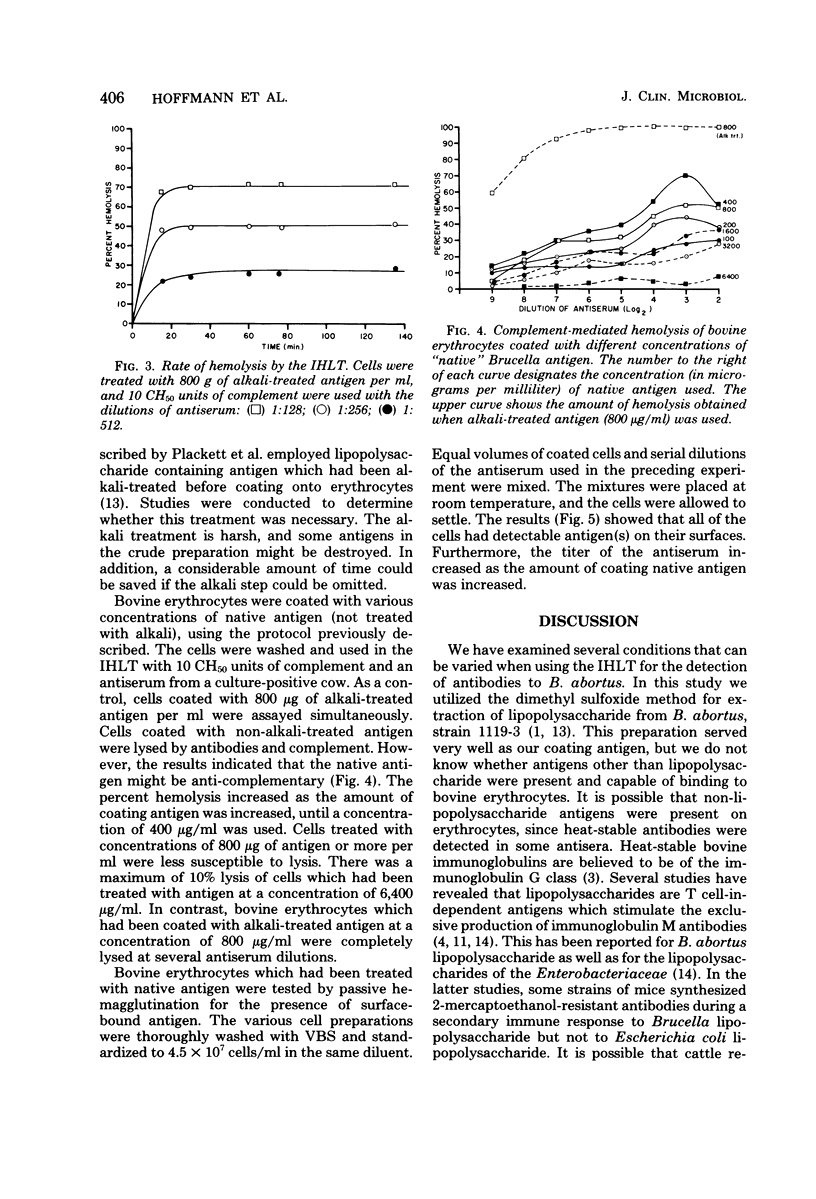
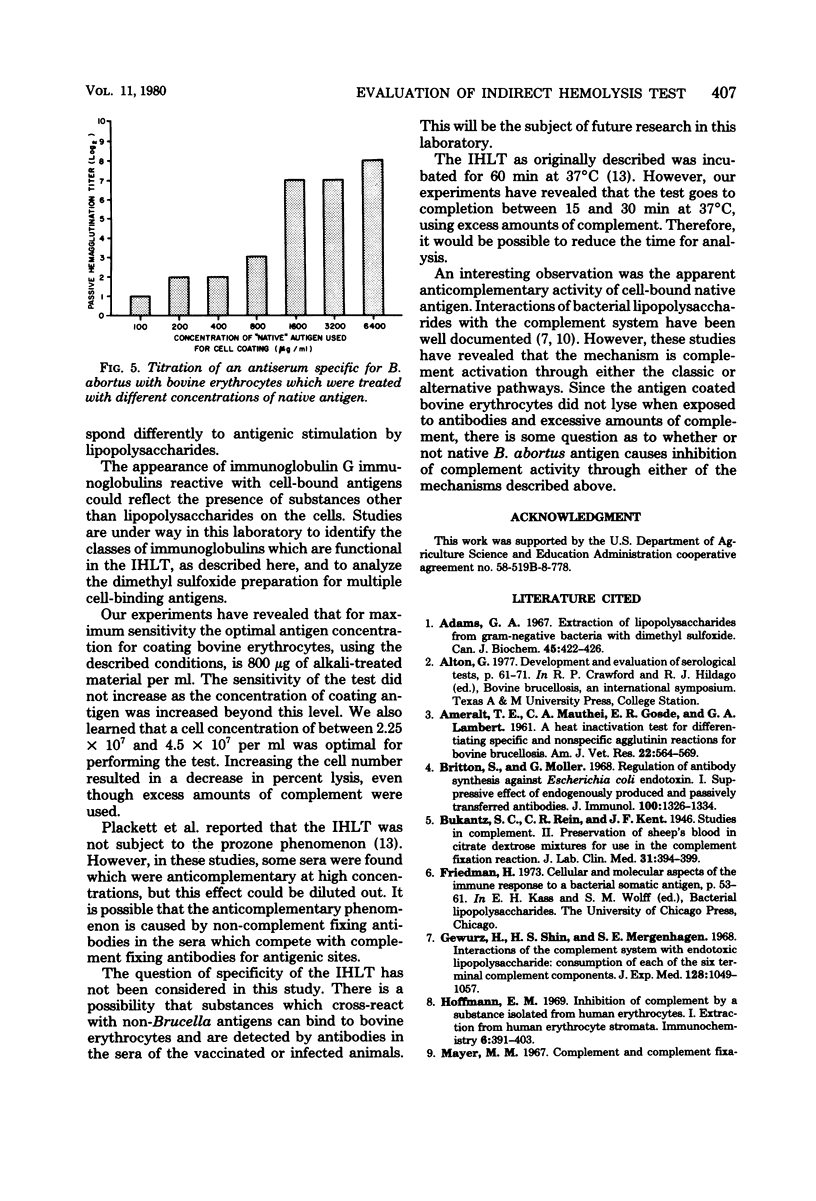
Some conditions were examined for performing the indirect hemolysis test for bovine brucellosis. An antigen extracted by using dimethyl sulfoxide was used for all of the assays. Optimal results were obtained by using bovine erythrocytes coated with alkali-treated antigen at a concentration of 800 micrograms/ml. Exceeding this level did not give greater sensitivity. The sensitivity of the test could be decreased by increasing the number of coated erythrocytes used in the test. Evidence was also provided for the presence of heat-labile antibodies in the sera of vaccinated cattle. Heat treatment (58 degrees C for 50 min) caused a reduction in titer of all sera tested. It was also shown that lysis of erythrocytes was complete in less than 60 min. Therefore, it would be possible to reduce the time needed for analysis. Non-alkali-treated ("native") antigen would bind to bovine erythrocytes, but it was less effective in the test than alkali-treated material. Erythrocytes coated with relatively large amounts of the native antigen were less suspectible to lysis than were cells which had been treated with lower concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMERAULT T. E., MANTHEI C. A., GOODE E. R., Jr, LAMBERT G. A heat-inactivation test for differentiating specific and non-specific agglutination reactions for bovine brucellosis. Am J Vet Res. 1961 May;22:564–569. [PubMed] [Google Scholar]
- Adams G. A. Extraction of lipopolysaccharides from gram-negative bacteria with dimethyl sulfoxide. Can J Biochem. 1967 Mar;45(3):422–426. doi: 10.1139/o67-050. [DOI] [PubMed] [Google Scholar]
- Britton S., Möller G. Regulation of antibody synthesis against Escherichia coli endotoxin. I. Suppressive effect of endogenously produced and passively transferred antibodies. J Immunol. 1968 Jun;100(6):1326–1334. [PubMed] [Google Scholar]
- Gewurz H., Shin H. S., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide: consumption of each of the six terminal complement components. J Exp Med. 1968 Nov 1;128(5):1049–1057. doi: 10.1084/jem.128.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. M. Inhibition of complement by a substance isolated from human erythrocytes. I. Extraction from human erythrocyte stromata. Immunochemistry. 1969 May;6(3):391–403. doi: 10.1016/0019-2791(69)90296-1. [DOI] [PubMed] [Google Scholar]
- Nicoletti P. Further evaluations of serologic test procedures used to diagnose brucellosis. Am J Vet Res. 1969 Oct;30(10):1811–1816. [PubMed] [Google Scholar]
- Plackett P., Cottew G. S., Best S. J. An indirect haemolysis test (IHLT) for bovine brucellosis. Aust Vet J. 1976 Mar;:136–140. doi: 10.1111/j.1751-0813.1976.tb05448.x. [DOI] [PubMed] [Google Scholar]
- STONE W. H., IRWIN M. R. The J substance of cattle. I. Developmental and immunogenetic studies. J Immunol. 1954 Dec;73(6):397–406. [PubMed] [Google Scholar]
- Spellman J. M., Reed N. D. Immune and mitogenic responses by BALB/c, C3H/HeJ, and nude mice to Brucella abortus bacterin and lipopolysaccharide. Infect Immun. 1979 May;24(2):371–378. doi: 10.1128/iai.24.2.371-378.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


