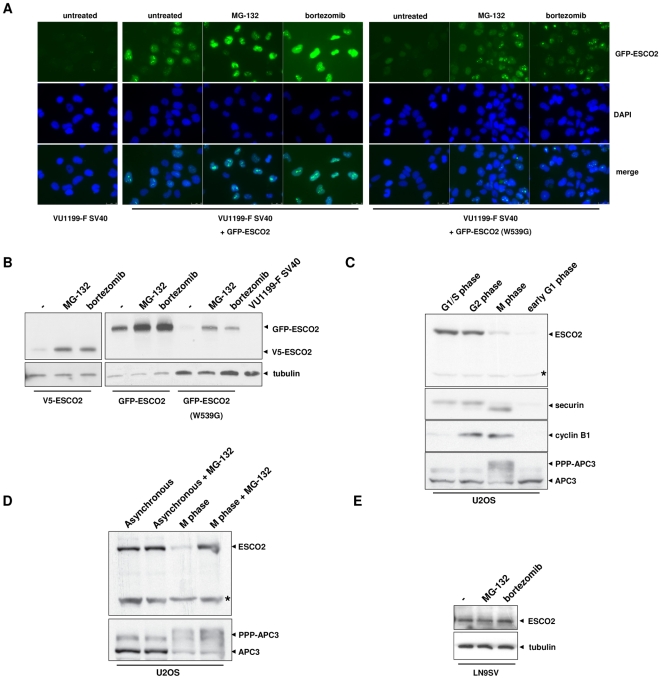
Figure 3. ESCO2 levels are regulated by proteasomal degradation.
(A) Fluoresence microscopy showing stabilization of ESCO2 protein by proteasome inhibitors in VU1199-F SV40 cells stably expressing wild type or mutant ESCO2. Cells were treated for 6 h with 50 µM MG-132 or 100 nM bortezomib and fixed with 100% cold methanol. Nuclei were stained with DAPI. (B) Ectopic ESCO2 is stabilized by proteasome inhibitors as shown by Western blotting. Cell lines were exposed to proteasome inhibitors for 6 h with 50 µM MG-132 or 100 nM bortezomib and analyzed for ESCO2 expression by Western blotting. Tubulin was used as loading control. (C) Endogenous ESCO2 levels in synchronized U2OS cells. Cells were arrested in G1/S phase by a thymidine block and released for 9 h to obtain cells in G2 phase. Mitotic cells were harvested by treatment with nocodazole followed by mitotic shake-off. These cells were released for 90 min to obtain early G1 phase cells. Cell lysates were analyzed for ESCO2, securin, cyclin B1 and APC expression by Western blotting. Asterisk indicates an aspecific band considered as loading control. (D) Proteasome inhibitor MG-132 stabilizes ESCO2 levels in M phase cells. U2OS cells were cultured for 4 h in the presence of 5 µM MG-132 before M phase cells were isolated. APC3 phosphorylation is shown as a control for mitotic cells. Asterisk indicates an aspecific band as loading control. (E) ESCO2 levels in unsynchronized wild type fibroblasts treated with proteasome inhibitors. LN9SV was treated for 6 h with 50 µM MG-132 or 1 µM bortezomib.