Abstract
Ribosome-inactivating proteins (RIPs) and sesquiterpenoid trichothecene mycotoxins are known to bind to eukaryotic ribosomes, inhibit translation and activate mitogen-activated protein kinases. Here we compared the capacities of the RIP ricin to promote 28S ribosomal RNA (rRNA) cleavage with that of the trichothecenes, deoxynivalenol (DON), and T-2 toxin (T-2). In a cell-free model, exposure to ricin at 300 ng/ml for 30 min depurinated yeast 28S rRNA, however, neither DON (≤ 4 μg/ml) nor T-2 (≤ 2 μg/ml) exhibited this N-glycosidase activity. Incubation of RAW 264.7 macrophages with ricin (20–320 ng/ml), DON (250–5000 ng/ml), or T-2 (2–80 ng/ml) for 6 h, however, generated 28S rRNA-specific products consistent with cleavage sites near the 3′ terminal end of murine 28S rRNA. Oligonucleotide extension analysis of treated RAW 264.7 cells revealed that ricin evoked 28S rRNA damage at one site in the α-sarcin/ricin (S/R)-loop (A4256) and two other sites (A3560 and A4045) in the peptidyl transferase center. Although DON or T-2 did not damage the S/R loop, these trichothecenes did promote cleavage at A3560 and A4045. In addition, incubation of the cells with ricin (≥ 20 ng/ml), DON (≥ 250 ng/ml), or T-2 (≥ 10 ng/ml) induced RNase activity as well as RNase L mRNA and protein expression. These data suggest that only ricin directly damaged 28S rRNA under cell-free conditions but that ricin, DON, and T-2 promoted intracellular 28S rRNA cleavage, potentially by facilitating the action of endogenous RNases and/or by upregulating RNase expression.
Keywords: cell culture; natural products; signal transduction; immunotoxicity, 285 rRna, RNase, mycotoxin
Ribosome-inactivating proteins (RIPs) from plants (e.g., ricin, abrin) (Hartley and Lord, 2004), fungi (e.g., α-sarcin) (Olmo et al., 2001), and bacteria (e.g., Shiga toxin) (Endo et al., 1988) interfere with eukaryotic ribosome peptidyl transferase function (Cai et al., 2000; Fordham-Skelton et al., 1991). In a process known as the ribotoxic stress response, RIPs activate mitogen-activated protein kinases (MAPKs) that mediate gene expression and apoptosis (Iordanov et al., 1997). The ribotoxic stress response is similarly evoked by antibiotics, which bind peptidyl transferase such as anisomycin, and by ultraviolet (UV) irradiation (Iordanov et al., 1998).
Although many downstream effects of the ribotoxic stress response have been delineated, the upstream mechanisms are only partially understood. It has been proposed that MAPK activation results directly from ribosomal damage (Iordanov et al., 1997). RIPs contain a RNA N-glycosidase domain that specifically depurinates adenine in the highly conserved sarcin/ricin (S/R) loop domain of eukaryotic 28S ribosomal RNA (rRNA) (Endo and Tsurugi, 1986). Cellular responses to UV radiation involving Jun N-terminal kinase 1 (JNK1) and nuclear factor-kappa B might similarly be a consequence of 28S rRNA damage (Devary et al., 1993; Iordanov et al., 1998). Accordingly, 28S rRNA cleavage appears to be a common critical event during ribotoxic stress that precedes MAPK activation and subsequent modulation of gene expression and apoptosis.
The trichothecene mycotoxins, a family of over 200 sesquitepenoids that are produced by Fusarium, Stachybotrys, and other fungi found in food and the environment, include some of the most potent translational inhibitors known (Grove, 2007). T-2 toxin (T-2) and deoxynivalenol (DON) are representative type A and B trichothecenes, respectively, that frequently contaminate cereal grains and are capable of dysregulating immune function and growth (Pestka and Smolinski, 2005). Trichothecenes rapidly induce MAPK phosphorylation in spleen and Peyer's patches of mice (Moon and Pestka, 2003; Zhou et al., 2003a) as well as in leukocyte cultures (Shifrin and Anderson, 1999; Yang et al., 2000). Trichothecene-induced p38 and ERK1/2 activation have been further demonstrated to modulate expression of genes associated with immune response, chemotaxis, inflammation, as well as induce apoptosis in clonal macrophage and monocyte cultures (Chung et al. 2003a, b; Moon and Pestka, 2002, 2003; Zhou et al., 2003b, 2005a, b). DON, T-2 and other trichothecenes can thus be included in the growing list of ribotoxic stressors (Pestka et al., 2004).
Although DON and T-2 are known to bind to the eukaryotic ribosome and inhibit translation (Ehrlich and Daigle, 1985, 1987), their capacity to promote rRNA damage has not been systematically addressed. Here, we employed cell-free and the RAW 264.7 clonal macrophage model to test the hypothesis that DON and T-2 exposure mediates cleavage of rRNA. The results suggest that although these trichothecenes did not possess the inherent enzymatic activity of ricin in a cell-free model, DON and T-2 evoked 28S rRNA cleavage in the macrophage, possibly by facilitating the action of constitutive and induced RNases.
MATERIALS AND METHODS
Chemicals.
DON and T-2 were purchased from Sigma-Aldrich (St Louis, MO). Ricin was obtained from Vector Labs, Inc. (Burlingame, CA). Other chemicals and media components were purchased from Sigma-Aldrich, except where noted.
rRNA depurination assay.
Yeast (Saccharomyces cerevisiae) was grown in YPD medium (1% [wt/vol] yeast extract, 2% [wt/vol] peptone, 2% [wt/vol] glucose, pH 6.5) to an A600 of 1.5–2.0. Cells were harvested by centrifugation and ribosomes isolated as described by Park et al. (2006). The ribosome pellet was resuspended in depurination buffer (100mM Tris-HCl, pH 7.2, 167mM KCl, and 100mM MgCl2), aliquoted, and stored at −80°C. The rRNA depurination assay was performed as described previously (Tumer et al., 1997). Briefly, 100 μg of purified ribosomes were suspended in a final volume of 100 μl of reaction buffer in the presence or absence of DON, T-2, or ricin. The mixtures were incubated for 30 or 60 min at 30°C. RNA was extracted with TRIZOL reagent (Invitrogen, Carlsbad, CA) and depurination analysis was conducted by incubation of RNA and 1M aniline acetate, pH 4.5 on ice for 30 min to induce cleavage at depurinated sites. RNA was precipitated with ethanol and depurination and was confirmed by the detection of a 370 nucleotide (nt) fragment following electrophoresis under denaturing conditions on a 4% (wt/vol) acrylamide gel.
Macrophage cell culture.
RAW 264.7 (ATCC, Rockville, MD), a mouse macrophage cell line, was cultured in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), streptomycin (100 μg/ml) and penicillin (100 U/ml) at 37°C in a humidified atmosphere with 5% CO2. Macrophage cell number and viability were assessed by trypan blue dye exclusion using a hematocytometer. Prior to exposure of toxins, cells (2.5 × 106/ml) were seeded and cultured in 100-mm tissue culture plates for 24 h to achieve approximately 80% confluency.
Northern blot analysis.
Northern blot analysis was performed by a modification of a previously described procedure (Li et al., 2003). cDNA probes were prepared by PCR amplification with primer pairs specific for murine 28S rRNA: 5′-gcggagagccgttcgtcttg-3′ and 5′-acaaacccttgtgtcgagggc-3′ and for murine 18S rRNA: 5′-agagggacggccgggggcat-3′ and 5′-tgggaataacgccgggccatc-3′. The resultant probes targeted positions G4515 to T4716 located at the 3′ end of mouse 28S RNA and A921 to A1120 located midway between the 5′ and 3′ ends of mouse 28S RNA. Probes were labeled with biotin-dUTP (deoxy-uridine triphosphate) (Roche Diagnostics GmbH, Mannheim, Germany). Total RNA was extracted using TRIZOL reagent and 10 μg separated in a 1.5% (wt/vol) agarose gel with formaldehyde. The gel was blotted onto a Biodyne membrane (Pall Gelman Laboratory, Ann Arbor, MI) and the membrane then UV cross-linked. Following prehybridization for 1 h at 48°C, the membranes were hybridized at 68°C overnight with biotinylated probes in Quickhyb solution (Stratagene, La Jolla, CA) containing 200 μg/ml of herring sperm DNA. Blots were washed twice with 2× 0.3M saline, 0.03M sodium citrate (SSC) containing 0.1% (wt/vol) sodium dodecyl sulfate (SDS) at room temperature and once for 15 min with 0.1× SSC containing 0.1% (wt/vol) SDS at 50°C. Membranes were blocked with Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE) containing 1% (wt/vol) SDS at 25°C for 1 h. Biotin-containing bands were identified by incubation with streptavidin-IRDye 800CW (Li-Cor Biosciences) and scanning on a Odyssey Infrared Imaging System (Li-Cor Biosciences).
Oligonucleotide extension.
28S rRNA cleavage sites were identified by oligonucleotide extension with Thermoscriptase (Invitrogen) and 5′6-carboxyfluorescein (FAM) labeled primers (Table 1) as described by Fekete et al. (2003). Briefly, 1 μg of purified RNA was mixed with 10nM FAM-labeled primer in 12 μl of H2O. Samples were heated to 90°C for 3 min, cooled on ice for 5 min and then incubated at 56°C for 20 min. First strand cDNA synthesis was performed by adding 8 μl of master mix containing 4 μl of 5× Thermoscriptase buffer, 1 μl of 10mM deoxy-nucleotide triphosphate (dNTP), 1 μl of 100mM dithiotreitol, 1 μl of Thermoscriptase and 1 μl of RNASIN RNase inhibitor and incubating at 70°C for 60 min. Following the addition of 0.1 volume of 3M sodium acetate and three volumes of absolute ethanol, cDNA was precipitated for 1 h at −80°C and pelleted by centrifugation at 20,000 × g for 25 min. The molecular size and relative quantities of the extension fragments were estimated according to shift distance of labeled DNA ladders and heights of fragment peaks, respectively, using an ABI 377 DNA Sequencer and GeneScan Analysis Software at Genewiz Inc. (South Plainfield, NJ). A 3 nt correction factor was used to correct for size overestimation due to FAM label (Fekete et al., 2003).
TABLE 1.
Primer Sequence for 28S rRNA Reverse Transcription and Cleavage Sites Identified by Primer Extension following Exposure of RAW 264.7 Cells with DON, T-2, or Ricin
| Primer name | Sequence and position | Target site |
Location | ||
| DON | T-2 | Ricin | |||
| P1 | ctcggccaagcactacacca | — | — | A4256 | S/R loop |
| 4300–4281 | |||||
| P2 | gccgacatcgaaggatc | A4045 | A4045 | A4045 | Anisomycin site |
| 4107–4091 | |||||
| P3 | cttattctacacctctca | A3560 | A3560 | A3560, G3559 | Anisomycin site |
| 3622–3605 | |||||
RNase activity.
Treated cells were washed, suspended in PBS, sonicated, centrifuged and the supernatant kept at −80°C until analysis. RNase activity was determined with an RNAseAlert QC kit (Ambion, Austin, TX) according to manufacturer's instructions.
Quantitative real-time PCR.
Primer pairs were designed using Gen Bank sequences as follows: RNase L, 5′-ctgtccggctggcagatt-3′ (forward), 5′-caagtctctcctgaccatctgtga-3′ (reverse), RNase 6, 5′-tgctctcagcctaagggtctct-3′ (forward), 5′-gcatggttgacgacttgtctgt-3′ (reverse). For each assay, 1 μg of total RNA was denatured by incubation at 70°C for 10 min with 250 ppm Oligo (dT)12–15 primer (Invitrogen). Real-time PCR was performed using ABI SYBR green PCR core kits (Applied Biosystems, Foster City, CA) and an ABI prism 7700 Sequence Detector (Kinser et al. 2005). Reaction mixtures (12.5 μl) contained 1μM primer pairs, 3mM Mg+2, 0.8mM dNTP mixture, 6 ng cDNA template and 0.075 μl of Taq Gold DNA polymerase (ABI). Reactions were incubated 95°C for 5 min then subjected to 40 two-step thermal cycles of 15 denaturation at 95°C, 60-s primer annealing and 60-s extension at 60°C. Real-time measurements were made and a threshold cycle (Ct) value for each sample was calculated. All samples were analyzed in duplicate. Positive controls containing 101, 102, 103, 104,105 106, 107, 108 copies/ml of target gene were used for standard curve preparation.
RNase L protein detection.
Whole-cell lysates were prepared as described previously (Li et al., 2003) and 60 μg aliquots were electrophoresed in 12% (wt/vol) polyacrylamide gels and transferred to a polyvinylidene fluoride transfer membrane (Millipore, Chelmsford, MA). Blots were sequentially incubated with goat anti-mouse RNase L (T16, Santa Cruz Biotechnology, Santa Cruz, CA) and IRDye 800CW-labeled donkey anti-goat IgG and then scanned with a Li-Cor Odyssey scanner.
Statistics.
Data were analyzed using Sigma Stat 3.11 (Jandel Scientific, San Rafael, CA). The Kruskal-Wallis one-way ANOVA on ranks with the Student-Newman-Keuls post test were used for comparison of multiple groups of data. Data sets were considered significantly different when p < 0.05.
RESULTS
DON and T-2 Do Not Possess RNA N-Glycosidase Activity
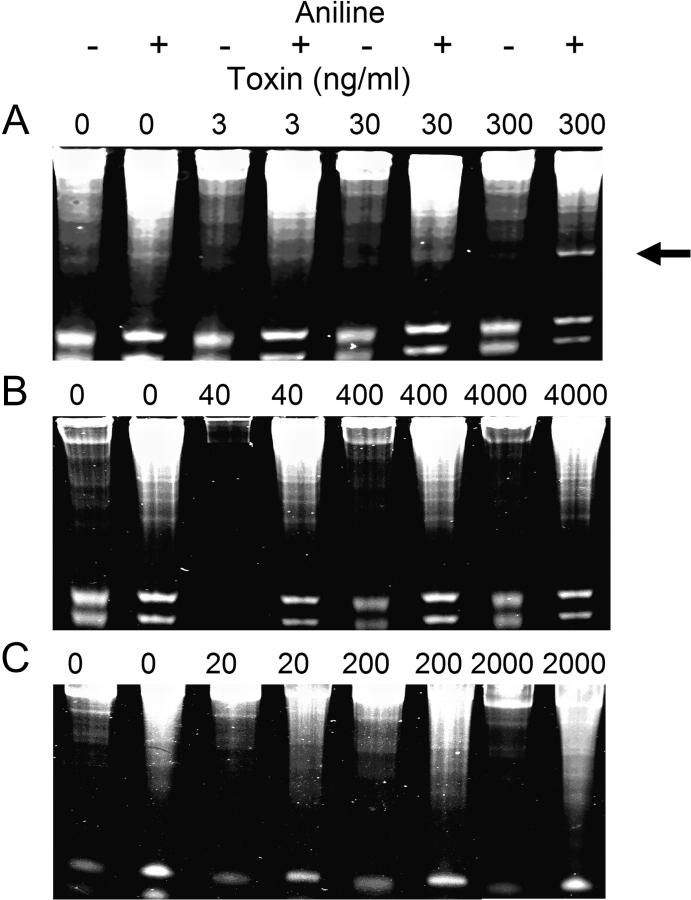
Type II RIPS possess RNA N-glycosidase activity that acts on 28S rRNA in eukaryotic ribosomes and is detectable under cell-free conditions (Park et al., 2006). To determine whether DON or T-2 have inherent glycosidase activity, their effects were compared with those of ricin in an in vitro depurination assay employing purified yeast ribosomes. Aniline treatment of rRNA from yeast ribosomes incubated with ricin at 300 ng/ml but not 3, 30 ng/ml generated a 370 nt fragment (Fig. 1A) that is diagnostic for this toxin's previously reported glycosidase activity. In contrast, incubation of yeast ribosomes with DON (40–4000 ng/ml) (Fig. 1B) or T-2 (20–2000 ng/ml) (Fig. 1C) caused neither depurination nor fragmentation of rRNA.
FIG. 1.
Ricin evokes depurination of yeast 28S rRNA in vitro. Yeast ribosomes were treated with indicated concentrations of (A) ricin, (B) DON, or (C) T-2 for 30 min at 37°C. RNA was extracted, treated with aniline acetate for 30 min at 30°C, and subjected to electrophoresis on acrylamide. Arrow indicates aniline-dependent 370 nt 28S rRNA fragment induced by ricin (300 ng/ml). Results are representative of six separate experiments.
DON, T-2, and Ricin Induce rRNA Fragmentation in RAW 264.7 Cells
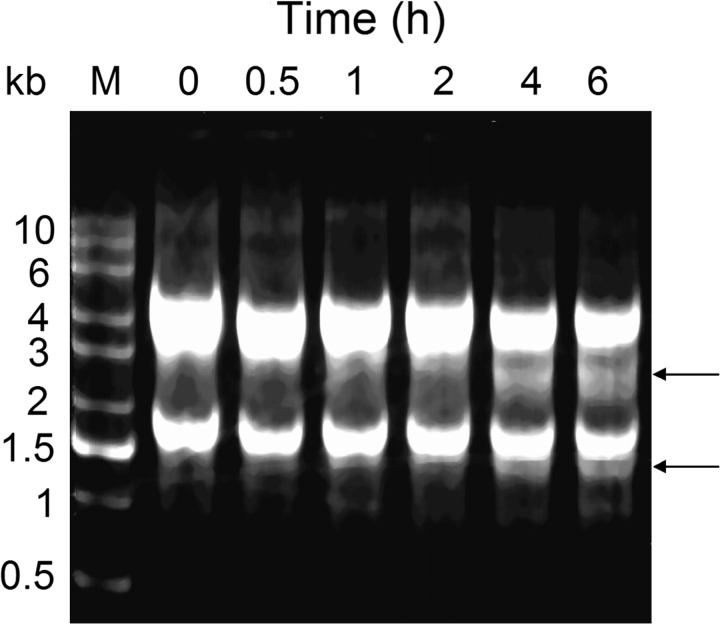
Incubation of RAW 264.7 cells with 2 μg/ml of DON for 6 h caused a time-dependent degradation of the 28S rRNA (Fig. 2). Concurrent with decreased 28S rRNA band intensities at 4 and 6 h, a 2–3 kb band and a 1–1.5 kb appeared. Based on size and abundance, these bands were consistent with being a cleavage products of the 28S rRNAs.
FIG. 2.
DON induces rRNA degradation in RAW 264.7 cells. Cells were treated with DON (2 μg/ml) for indicated time intervals. Total RNA was isolated and subjected to agarose gel electrophoresis under denaturing conditions. Arrows indicate 28S rRNA cleavage fragments. Results are representative of four separate experiments.
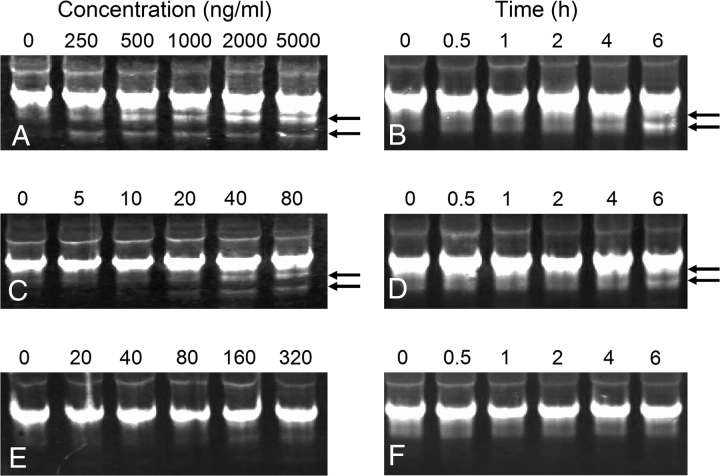
Northern blot analyses using biotin-labeled dUTP probes for 18S and 28S rRNAs were employed to verify trichothecene-induced rRNA cleavage in RAW 264.7 cells and to compare these effects to those of ricin. DON (Figs. 3A and 3B) and T-2 (Figs. 3C and 3D) induced concentration- and time-dependent fragmentation of 18S rRNA, however, specific 18S rRNA cleavage products were not similarly evident in cells treated with ricin (Figs. 3E and 3F).
FIG. 3.
Induction of 18S rRNA cleavage in RAW 264.7 cells by DON, T-2 and ricin. Cells were treated with DON (A), T-2 (C), or ricin (E) at indicated concentrations for 6 h or with DON (2 μg/ml) (B), T-2 (80 ng/ml) (D), or ricin (160 ng/ml) (F) for indicated time intervals. Northern blots were performed on total RNA with biotin-dUTP labeled probes and streptavidin-labeled IRDye 800CW and analyzed for infrared fluorescence. Arrows indicate 18S rRNA cleavage products. Results are representative of two separate experiments.
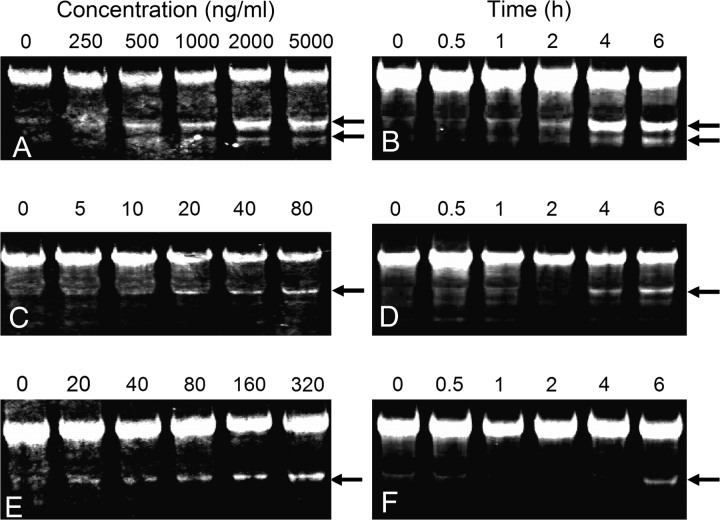
Incubation with DON (250–2000 ng/ml) for 6-h induced concentration-dependent fragmentation of 28S rRNA in RAW 264.7 cells with two fragments being observed (Fig. 4A). Treatment with 2 μg/ml DON for as little as 1 h induced similar fragmentation (Fig. 4B). The observed fragments corresponded to degraded 28S rRNA at the 3′ terminus with approximate sizes of 1200 and 1500 nt based on comparison to RNA ladders (not shown). In comparison, a single 28S rRNA cleavage product was primarily seen in RAW 264.7 cells incubated with 20–80 ng/ml T-2 for 6 h (Fig. 4C) or with 80 ng/ml T-2 for 4 h (Fig. 4D). Treatment with 20–320 ng/ml ricin for 6 h (Fig. 4E) or 160 ng/ml for 4 h (Fig 4F) induced a single fragment. The T-2- and ricin-induced fragments corresponded to cleavage at approximately 1500 nt from 3′ terminal end of the 28S rRNA. The sizes of the 28S rRNA fragments generated by the three toxins were consistent with cleavage in the peptidyl transferase center. Additional smaller fragments with sizes ranging from 600 to 1200 nt were observed after 6- or 8-h exposure to the highest concentrations of the three toxins (data not shown).
FIG. 4.
Induction of 28S rRNA cleavage in RAW 264.7 cells by DON, T-2 and ricin. Cells were treated with DON (A), T-2 (C), or ricin (E) at indicated concentrations for 6 h or with DON (2 μg/ml) (B), T-2 (80 ng/ml) (D), or ricin (160 ng/ml) (F) for indicated time intervals and analyzed as described in Figure 3 legend. Arrows indicate 28S rRNA cleavage products. Results are representative of four separate experiments.
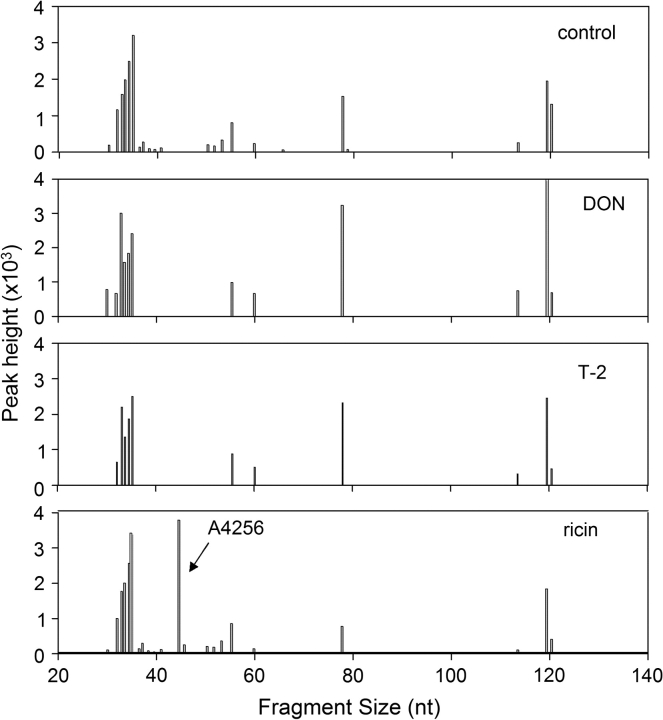
DON, T-2 and Ricin Cause Cleavage at Different Sites of 28S rRNA
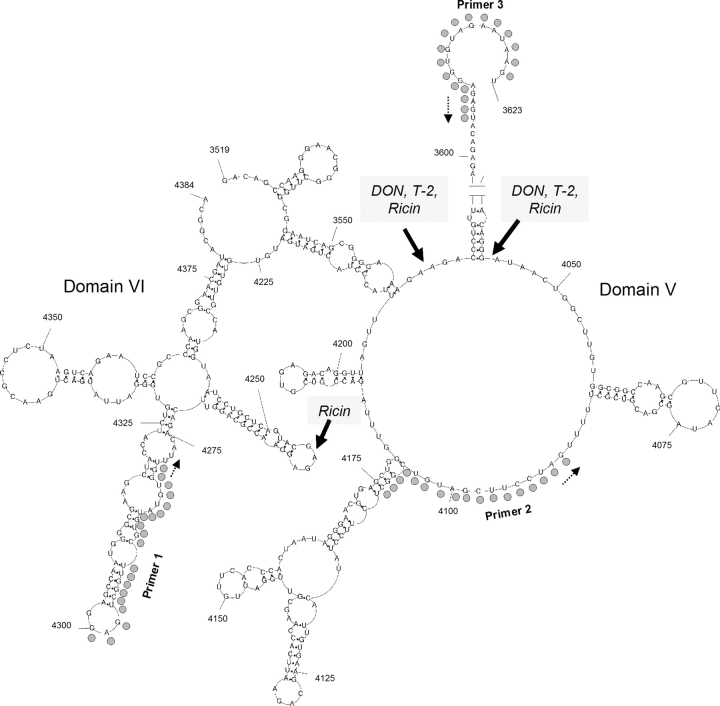
Oligonucleotide extension was used to localize lesions in the peptidyl transferase region of 28S rRNA. Following incubation of RAW 264.7 cells with ricin (160 ng/ml), DON (1 μg/ml) or of T-2 (40 ng/ml) for 4 h, reverse transcription reactions using FAM-labeled primers were performed on total RNA and the resultant fragments were size-separated and quantified. When potential cleavage sites located between nt 4160 and 4300 of 28S rRNA were assessed (P 1, Table 1), ricin was found to induce a 28S rRNA cleavage product that mapped to A4256 (Fig. 5), which is located in the previously described S/R loop of domain VI (Fig. 6). Cleavage at this site was detectable in ricin-treated cells as early as 1 h (data not shown). In contrast, DON or T-2 exposure did not cleave the 28S rRNA in the 4164–4304 nt region (Fig. 5). The stabilities of rRNA stem loops might have also contributed to inefficient read-through by Thermoscriptase, which contributed to clusters of toxin-independent strong stops. These were observed immediately downstream of P1 approximately located 10 nt upstream and downstream of A4256 and 80 and 120 nt from the primer start.
FIG. 5.
Oligonucleotide extension analysis of toxin-induced cleavage of 28S rRNA S/R loop in RAW 264.7 cells. Cells were incubated with DON, T-2, and ricin RAW cells were treated with DON (1000 ng/ml), T-2 (80 ng/ml), and ricin (160 ng/ml) for 4 h. Reverse transcription reaction was performed on total RNA using FAM-labeled primer 1–specific for mouse 28S rRNA from 4300 to 4281 (depicted in Fig. 6). Resultant cDNA extension fragments were sized and quantified. Results are representative of two separate experiments.
FIG. 6.
Peptidyl transferase target sites of ricin, DON and T-2. Secondary structure of the peptidyl transferase region of mouse rRNA based on Michot et al. (1984) using base pairings described by Larsson et al. (2002). Structure was drawn with PseudoViewer web application (http://pseudoviewer.inha.ac.kr/) developed by Byun and Han (2006). Filled circles indicates primer sites and dotted arrow show direction of primer extension. Solid arrows indicate targets for ricin, DON and T-2 identified by oligonucleotide extension.
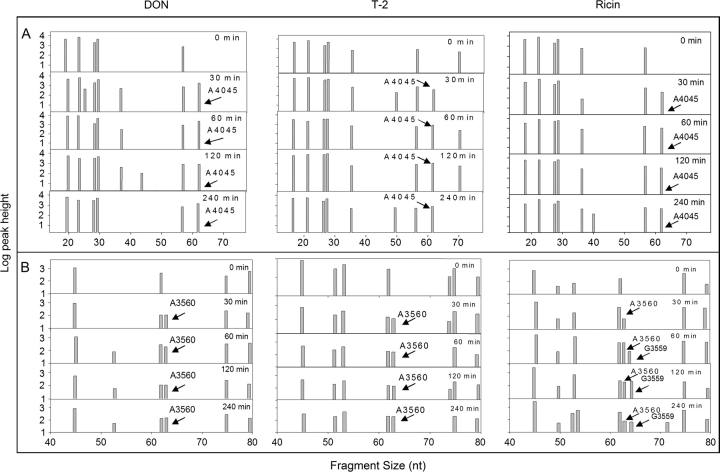
When two other primers (P2 and P3, Table 1) were used to scan the 28S rRNA peptidyl transferase center, additional cleavage sites were identified for the three toxins (Figs. 7A and 7B). DON, T-2, and ricin induced cleavage products of sizes that mapped approximately to A3560 and A4045. Both A3560 and A4045 are located the central loop region of domain V of 28S rRNA peptidyl transferase center (Fig. 6), a putative site of action region for the antibiotics anisomycin, chloramphenicol and blasticidin S (Iordanov et al., 1997).
FIG. 7.
Oligonucleotide extension analysis of toxin-induced 28S rRNA peptidyl transferase cleavage in RAW 264.7 cells. Cells were treated with DON (1000 ng/ml), T-2 (80 ng/ml), and ricin (160 ng/ml) for 0, 0.5, 1, 2, and 4 h. Reverse transcription reaction was performed on total RNA using FAM-labeled (A) primer 2 specific for mouse 28S rRNA specific, from 4107 to 4091) or (B) primer 3 specific for mouse 28S rRNA from 3622 to 3605 (depicted in Fig. 6). Resultant cDNA extension fragments were sized and quantified. Results are representative of two separate experiments.”
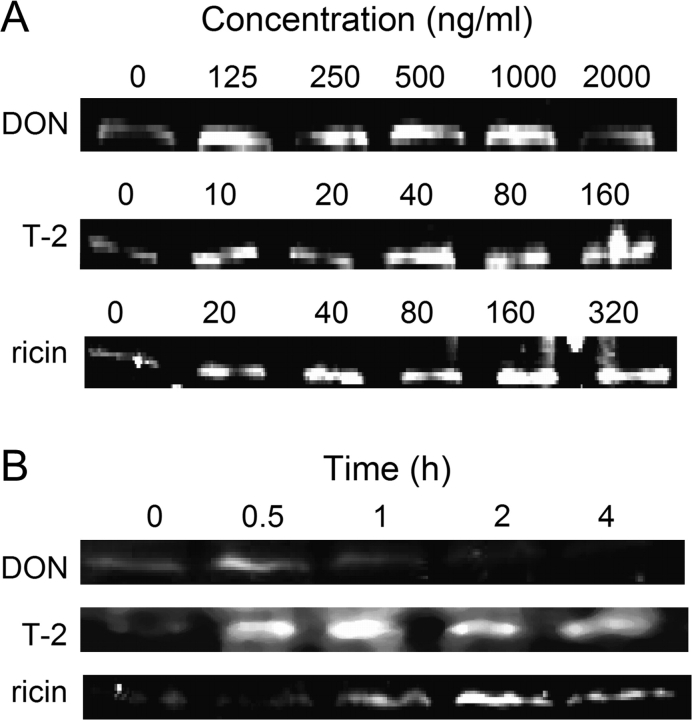
DON, T-2, and Ricin Alter RNase Activity of RAW Cells
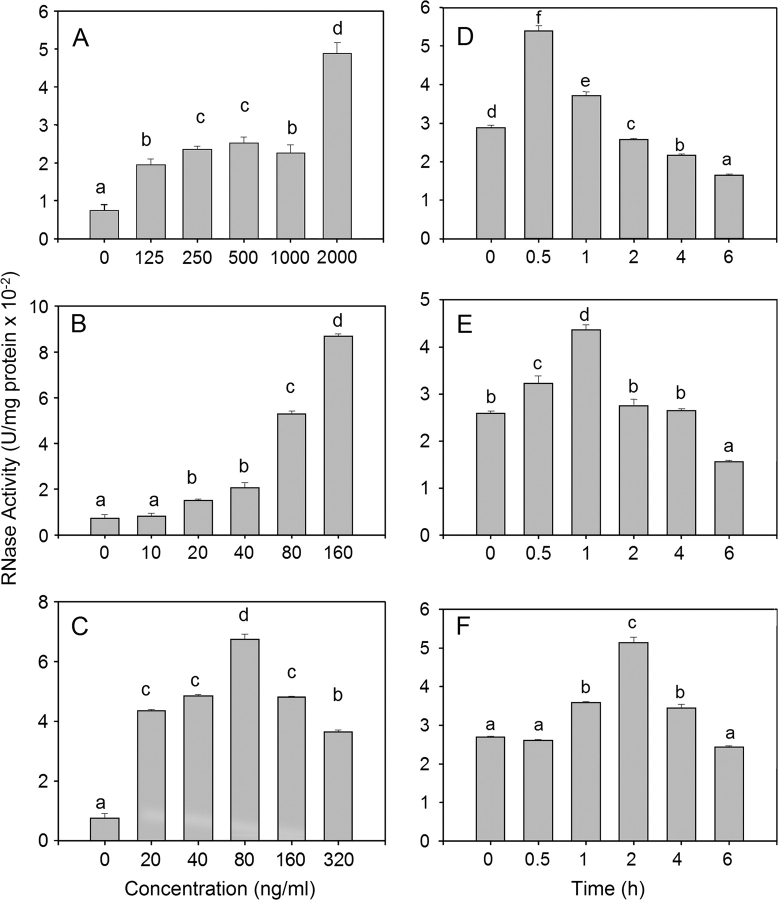
Because DON and T-2 induced 28S rRNA cleavage in RAW 264.7 cells, but did not possess inherent RNA N-glycosidase activity, their effects might be facilitated by constitutive or inducible RNases. The effects of DON, T-2, and ricin exposure in RAW 246.7 cells on RNase activity were therefore compared. Total RNase activity was significantly increased following 1-h incubation with 125–2000 ng/ml of DON (Fig. 8A), 20–160 ng/ml of T-2 (Fig. 8B), and 20–320 ng/ml of ricin (Fig. 8C) as compared with incubating with the vehicle for 1 h. Peak RNase activities were observed after 0.5 or 1 h of exposure to DON (1000 ng/ml) (Fig. 8D) or T-2 (160 ng/ml) (Fig. 8E) and 1, 2, and 4 h exposure to ricin (320 ng/ml) (Fig. 8F). RNAse activities decreased to control levels or below 2 h after DON or T-2 exposure and 6 h after ricin exposure. Thus, DON, T-2, and ricin treatments appeared to transiently increase RNase activity in RAW 264.7 cells.
FIG. 8.
DON, T-2, and ricin induce RNase activity in RAW 264.7 cells. Cells were treated with DON (A), T-2 (C), or ricin (E) at indicated concentrations for 1 h or with DON (1000 ng/ml) (B), T-2 (160 ng/ml) (D), or ricin (320 ng/ml) (F) for indicated time intervals Total cellular proteins were extracted and RNase activity determined. Data are means ± SEM (n = 6) pooled from three separate experiments. Bars without same letter differ (p < 0.05).
DON, T-2, and Ricin Induce RNase Expression
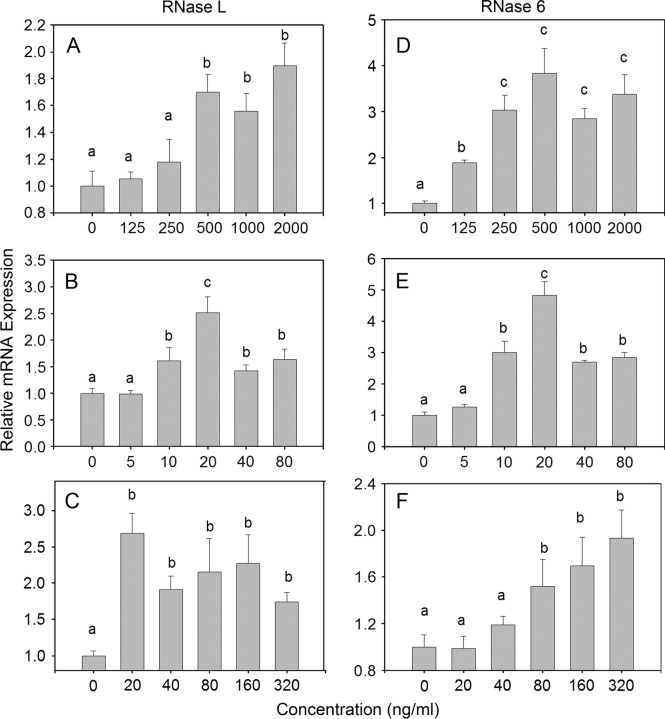
The effects of DON, T-2, and ricin on expression of two representative RNase mRNAs in RAW 264.7 cells were assessed by real time polymerase chain reaction. Both RNase L (Figs. 9A–C) and RNase 6 mRNA (Figs. 8D–F) expression were induced by of DON (125–2000 ng/ml), T-2 (10–80 ng/ml), and ricin (80–320 ng/ml) in a concentration-dependent fashion.
FIG. 9.
DON, T-2, and ricin concentration-dependently modulate RNase mRNA expression in RAW 264.7 cells. Cells were treated with DON, (A, D) T-2, (B, E) and ricin (C, F) for 2 h at the indicated concentrations. Total RNA was analyzed for RNase 6 and RNase L expression by real-time PCR. Data are means ± SEM (n = 8) pooled from two separate experiments. Bars without same letter differ (p < 0.05).
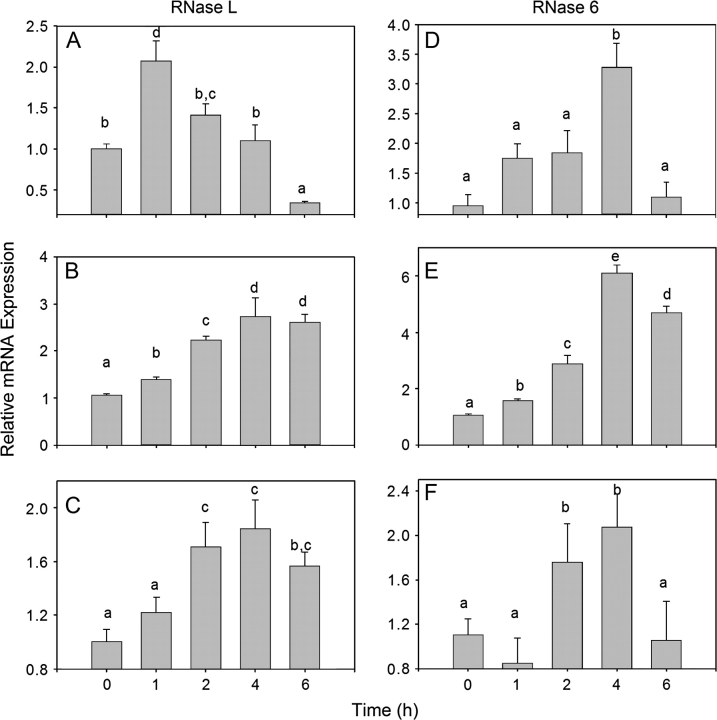
When the kinetics of toxin-induced RNase L and RNAse 6 mRNA expression were determined, RNase L mRNAs were observed to peak at 1 h, 2–6 h, and 2–6 h after exposure to DON (1000 ng/ml) (Fig. 10A), T-2 (80 ng/ml) (Fig. 10B), and ricin (320 ng/ml) (Fig. 10C), respectively. All three of the toxins induced peak RNase 6 mRNA expression 4 h after treatment (Figs. 10D–F).
FIG. 10.
DON, T-2, and ricin time-dependently alter RNase mRNA expression in RAW 264.7 cells. Cells were treated with DON (1000 ng/ml), (A, D) T-2 (160 ng/ml), (B, E) and ricin (320 ng/ml) (C, F) for indicated time interval. Total RNA was analyzed for RNase 6 and RNase L expression analyzed by real-time PCR. Data are means ± SEM (n = 8) pooled from two separate experiments. Bars without same letter differ (p < 0.05).
RNase L protein expression was assessed by Western blotting DON (≥ 125 ng/ml), T-2 (≥ 40 ng/ml), and ricin (≥ 160 ng/ml) (Fig. 11A) induced RNase L expression after 1 h. RNase L protein expression was induced by DON (1 μg/ml) from 0.5 to 2 h. T-2 (80 ng/ml) from 0.5 to 4 h and ricin (160 ng/ml) from 1 to 4 h (Fig. 11B). Thus, upregulation RNase L protein by the three toxins appeared to correspond to increased RNase L mRNA.
FIG. 11.
DON, T-2 and ricin altered RNase L protein expression in RAW 264.7 cells. Cells were treated with indicated concentration of DON, ricin, or T-2 for 1 h or with (A) 500 ng/ml of DON, 160 ng/ml of ricin, or 80 ng/ml of T-2 for indicated time interval (B). RNase L protein was probed by Western blotting. Data were representative of four separate experiments.
DISCUSSION
Although it is well established that trichothecene mycotoxins bind to eukaryotic ribosomes and inhibit translation, the mechanisms by which they cause MAPK-driven gene expression and apoptosis are not completely understood (Pestka et al., 2004). A proposed central mechanism for MAPK activation by ribotoxic stressors is cleavage of 28S rRNA at the peptidyl transferase center (Iordanov et al., 1997). This region encompasses the site of aminoacyl-tRNA binding, peptidyl transferase activity, and ribosomal translocation during translation (Uptain et al., 1997). The macrophage studies detailed here provide evidence that ricin induced rRNA damage in the S/R loop of domain VI as well as two other target sites (A3560, A4045) located the central loop region of domain V of 28S rRNA peptidyl transferase center whereas DON and T-2 acted only on these latter two sites (Fig. 6). Although damage of 28S rRNA can result from the direct action of enzymatically active toxins such as ricin, it appears possible that other ribotoxins might promote 28S rRNA cleavage by binding and making the RNA available to constitutive or inducible cellular RNases.
Type 2 RIPs such as ricin contain two peptide chains, A and B. The A chain possesses activity of RNA N-glycosidase which specifically depurinates a single adenine residue located near the 3′ terminal end of the 28S rRNA of the 60S ribosomal subunit at S/R loop (Endo and Tsurugi, 1986; Hartley and Lord, 2004; Olmo et al., 2001; Silva et al., 2004). This modification has been suggested to induce a conformational change that prevents binding of elongation factor 2 to the ribosome, thereby causing translational arrest (Olivieri et al., 1996). As expected, ricin exhibited glycosidase activity in a cell-free depurination assay described here. Northern blotting confirmed that ricin cleaved yeast 28S rRNA to yield a fragment consistent with its targeting of the S/R loop; this target was verified in the macrophage by oligonucleotide extension.
Although high concentrations of DON and T-2 did not directly depurinate or cleave 28S rRNA under cell-free conditions, these toxins induced 18S and 28S rRNA cleavage in RAW 264.7 cells, which is suggestive of an indirect, toxin-facilitated mechanism. Primer extension revealed that the trichothecenes targeted two sites, A3560 and A4045, located in the 28S rRNA peptidyl transferase center, specifically within the central loop region of domain V. RNA footprinting has previously determined that T-2 interacts at a site on yeast 26S rRNA corresponding to mouse A3560 (Rodriguez-Fonseca et al., 1995). A3560 and A4045 also mapped closely to sites targeted by anisomycin, which, like DON, binds to 28S rRNA, inhibits peptidyl transferase activity and activates MAPK (Iordanov et al., 1997). Anisomycin can block binding of trichothecenes to ribosomes (Middlebrook and Leatherman, 1989a) as well as prevent their uptake by eukaryotic cells (Middlebrook and Leatherman, 1989b; Witt and Pestka, 1990). Binding of DON, T-2, and perhaps anisomycin at this site could disrupt 28S rRNA structure, making it accessible to endogenous or upregulated cellular RNases. Indeed, upregulated RNase activity observed after treatment with the three toxins corresponded to elevated RNase L and RNase 6 expression, both of which might contribute to or enhance 28S rRNA damage.
RNase L is an endonuclease with a broad range of functions that includes inhibition of protein synthesis, apoptosis induction and antiviral activity (Stark et al., 1998). RNase L expression has previously been shown to be induced by virus infection, double-stranded (ds) RNA [poly(I:C)], chemotherapeutic drugs (cisplatin, doxorubicin, vinblastine), hydrogen peroxide (H2O2), calcium chloride(CaCl2), and cytokines (tumor necrosis factor-1α, interferron-β) (Goswami et al., 2004; Li et al., 1998; Pandey et al., 2004; Scherbik et al., 2006; Ushijima et al., 1993). RNase L can inhibit protein synthesis via the degradation of mRNA (Clemens and Vaquero, 1978) and/or rRNA (Hovanessian et al., 1979; Silverman et al., 1983; Wreschner et al., 1981). Activation of RNase L in cells has been previously demonstrated to cause of 28S rRNA cleavage, leading to a cellular stress response and activation of the JNK pathway (Iordanov et al., 2000; Li et al., 2004).
RNase 6 is a member of RNase A superfamily which share invariant structural and catalytic elements and enzymatic activity (Dyer et al., 2004). Little is known about the function of RNase 6, making it difficult to conjecture on a specific role for it in the 28S rRNA damage described here. Some RNase A family members, such as RNase 2, RNase 5 and RNase 7, appear to be involved in host defense (Dyer and Rosenberg, 2006). Consistent with that proposed role, our observations suggest minimally that RNase 6 can be upregulated in macrophage following stress induction.
Only two RNases were assessed in this study. Oligonucleotide extension suggested that the three toxins induced damage to the central loop region of domain V of 28S rRNA within 30 min. Although RNase activity increased in as little as 30 min after addition of DON and T-2 the cultures and 1 h after ricin addition, induction of maximum RNase L and RNase 6 mRNA expression sometimes took hours. Thus the importance of induction of these latter two RNases to central loop damage might be questioned because the possibility exists that constitutive RNases and/or other induced RNase isoform mediates this damage. Indeed, induction of RNase L and RNAse 6 mRNA might actually be a result of the stress response initiated by toxin-facilitated 28S rRNA cleavage by constitutive RNases.
Critical questions remain concerning the linkages existing between ribotoxin-induced 28S rRNA damage and downstream MAPK-driven toxic effects. dsRNA-activated protein kinase (PKR) is a serine/threonine kinase known to localize to the ribosome (Zhu et al., 1997; Wu et al., 1998). This enzyme and contains a specific dsRNA-binding motif that facilitates its activation (Williams, 2001). PKR phosphorylates eukaryotic initiation factor eIF-2α, thereby throttling down protein synthesis (Galabru and Hovanessian, 1987; Hartley and Lord, 2004). dsRNA also induces PKR-dependent expression of RNase L (Iordanov et al., 2000). Our laboratory has demonstrated that PKR mediates DON-induced MAPK activation (Zhou et al., 2003b). PKR-deficient U937 monocytes exhibit reduced induction of p38, JNK, and ERK phosphorylation by DON. Relative to p38, PKR is known to interact with apoptosis signaling kinase 1 (Takizawa et al., 2002) and mitogen-activated kinase 6 (Silva et al., 2004), both of which can drive p38 phosphorylation. Recently, PKR has been shown to form a functional complex with p38 (Alisi et al., 2008).
It is tempting to speculate that trichothecenes and other ribotoxins alter 28S rRNA tertiary structure sufficiently for it to bind and activate ribosomal-bound PKR and that this subsequently drives p38 phosphorylation. One possibility is that an altered tertiary structure resulting from ribotoxin binding might unmask sufficient ds-rRNA from the ribosome to activate PKR. An alternative possibility is that RNAse-mediated rRNA cleavage per se might render a portion of dsRNA immediately accessible to PKR. In support of the latter contention, the observed targeting of two sites in the central loop region of domain V of 28s rRNA by DON, T-2, and ricin could generate an accessible dsRNA containing an exposed 5′ end. The observations that lesions at A3560 and A4045 were observable within 30 min after trichothecene treatment, is consistent with previously reported rapid activation of MAPKs (Zhou et al., 2003a). In the future, it will be important to ascertain whether such a 28S rRNA lesion is sufficient to drive activation of PKR as well its downstream MAPK-mediated sequelae.
One limitation of this investigation was that we did not investigate 18S rRNA for specific lesion sites, despite the Northern blot data suggesting damage to this subunit RNA by the trichothecenes. A second limitation of this study, was that a small number of primers oriented around the peptidyl transferase center were used for oligonucleotide extension. Although this approach enabled focused scanning of relatively small sections (< 100 nt) of the 28S rRNA, it is possible that other lesions might have occurred that were outside the primer scan range. Expanded analysis for additional cleavage sites 18S and 28S rRNA is therefore warranted.
Taken together, the data presented here suggest that, in the macrophage, ricin induced rRNA damage in both the S/R loop and the central loop region of domain V of 28s rRNA peptidyl transferase center whereas DON and T-2 acted only on the domain V central loop. Cleavage of 28S rRNA at the S/R loop is likely to result from ricin's RNA N-glycosidase activity. We further propose that the trichothecenes and ricin interact with domain V, thereby facilitating cleavage of least two additional 28S rRNA sites by cellular RNAses. This is the first report to our knowledge of trichothecene-induced damage rRNA damage in a cell and it is not yet known whether the findings in the macrophage are generalizable to other cell types. Further investigations on the trichothecenes and other ribotoxic stressors are also necessary to uncover the specific relationships that exist among (1) rRNA target sites, (2) constitutive and induced RNases, (3) PKR activation, and (4) the ensuing MAPK responses.
FUNDING
Public Health Service Grants (ES03553 and DK058833) from the National Institutes for Health.
Acknowledgments
We thank Kaiyu He and Mary Rosner for technical assistance as well as Laura Schaefer, Rob Britton, and Jorge Vivanco for advice and suggestions.
References
- Alisi A, Spaziani A, Anticoli S, Ghidinelli M, Balsano C. PKR is a novel functional direct player that coordinates skeletal muscle differentiation via p38MAPK/AKT pathways. Cell Signal. 2008;20:534–542. doi: 10.1016/j.cellsig.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Byun Y, Han K. PseudoViewer: Web application and web service for visualizing RNA pseudoknots and secondary structures. Nucleic Acids Res. 2006;34(Web Server issue):W416–W422. doi: 10.1093/nar/gkl210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CQ, Guo H, Schroeder RA, Punzalan C, Kuo PC. Nitric oxide-dependent ribosomal RNA cleavage is associated with inhibition of ribosomal peptidyl transferase activity in ANA-1 murine macrophages. J. Immunol. 2000;165:3978–3984. doi: 10.4049/jimmunol.165.7.3978. [DOI] [PubMed] [Google Scholar]
- Chung YJ, Yang GH, Islam Z, Pestka JJ. Up-regulation of macrophage inflammatory protein-2 and complement 3A receptor by the trichothecenes deoxynivalenol and satratoxin G. Toxicology. 2003a;186:51–65. doi: 10.1016/s0300-483x(02)00605-4. [DOI] [PubMed] [Google Scholar]
- Chung YJ, Zhou HR, Pestka JJ. Transcriptional and posttranscriptional roles for p38 mitogen-activated protein kinase in upregulation of TNF-alpha expression by deoxynivalenol (vomitoxin) Toxicol. Appl. Pharmacol. 2003b;193:188–201. doi: 10.1016/s0041-008x(03)00299-0. [DOI] [PubMed] [Google Scholar]
- Clemens MJ, Vaquero CM. Inhibition of protein synthesis by double-stranded RNA in reticulocyte lysates: Evidence for activation of an endoribonuclease. Biochem. Biophys. Res. Commun. 1978;83:59–68. doi: 10.1016/0006-291x(78)90397-2. [DOI] [PubMed] [Google Scholar]
- Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- Dyer K, Rosenberg H. The RNase a superfamily: Generation of diversity and innate host defense. Mol. Divers. 2006;10:585–597. doi: 10.1007/s11030-006-9028-2. [DOI] [PubMed] [Google Scholar]
- Dyer KD, Rosenberg HF, Zhang J. Isolation, characterization, and evolutionary divergence of mouse RNAse 6: Evidence for unusual evolution in rodents. J. Mol. Evol. 2004;59:657–665. doi: 10.1007/s00239-004-2657-0. [DOI] [PubMed] [Google Scholar]
- Endo Y, Tsurugi K. Mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. Nucleic Acids Symp. Ser. 1986;17:187–190. [PubMed] [Google Scholar]
- Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 1988;171:45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich KC, Daigle KW. Protein synthesis by mammalian cells treated with C-3-modified analogs of the 12,13-epoxytrichothecenes T-2 and T-2 tetraol. Appl. Environ. Microbiol. 1985;50:914–918. doi: 10.1128/aem.50.4.914-918.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich KC, Daigle KW. Protein synthesis inhibition by 8-oxo-12,13-epoxytrichothecenes. Biochim. Biophys. Acta. 1987;923:206–213. doi: 10.1016/0304-4165(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Fekete RA, Miller MJ, Chattoraj DK. Fluorescently labeled oligonucleotide extension: A rapid and quantitative protocol for primer extension. Biotechniques. 2003;35:90–98. doi: 10.2144/03351rr01. [DOI] [PubMed] [Google Scholar]
- Fordham-Skelton AP, Taylor PN, Hartley MR, Croy RR. Characterisation of saporin genes: In vitro expression and ribosome inactivation. Mol. Gen. Genet. 1991;229:460–466. doi: 10.1007/BF00267470. [DOI] [PubMed] [Google Scholar]
- Galabru J, Hovanessian A. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J. Biol. Chem. 1987;262:15538–15544. [PubMed] [Google Scholar]
- Goswami BB, Kulka M, Ngo D, Cebula TA. Apoptosis induced by a cytopathic hepatitis A virus is dependent on caspase activation following ribosomal RNA degradation but occurs in the absence of 2′–5′ oligoadenylate synthetase. Antiviral Res. 2004;63:153–166. doi: 10.1016/j.antiviral.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove JF. The trichothecenes and their biosynthesis. Fortschr. Chem. Org. Naturst. 2007;88:63–130. [PubMed] [Google Scholar]
- Hartley MR, Lord JM. Cytotoxic ribosome-inactivating lectins from plants. Biochim. Biophys. Acta. 2004;1701:1–14. doi: 10.1016/j.bbapap.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Hovanessian AG, Wood J, Meurs E, Montagnier L. Increased nuclease activity in cells treated with pppA2′p5′A2′p5′ A. Proc. Natl. Acad. Sci. U. S. A. 1979;76:3261–3265. doi: 10.1073/pnas.76.7.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Paranjape JM, Zhou A, Wong J, Williams BR, Meurs EF, Silverman RH, Magun BE. Activation of p38 mitogen-activated protein kinase and c-Jun NH(2)-terminal kinase by double-stranded RNA and encephalomyocarditis virus: Involvement of RNase L, protein kinase R, and alternative pathways. Mol. Cell. Biol. 2000;20:617–627. doi: 10.1128/mcb.20.2.617-627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. Ribotoxic stress response: Activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol. Cell. Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Magun BE. Ultraviolet radiation triggers the ribotoxic stress response in mammalian cells. J. Biol. Chem. 1998;273:15794–15803. doi: 10.1074/jbc.273.25.15794. [DOI] [PubMed] [Google Scholar]
- Kinser S, Li M, Jia Q, Pestka JJ. Truncated deoxynivalenol-induced splenic immediate early gene response in mice consuming (n-3) polyunsaturated fatty acids. J. Nutr. Biochem. 2005;16:88–95. doi: 10.1016/j.jnutbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Larsson SL, Sloma MS, Nygard O. Conformational changes in the structure of domains II and V of 28S rRNA in ribosomes treated with the translational inhibitors ricin or alpha-sarcin. Biochim. Biophys. Acta. 2002;1577:53–62. doi: 10.1016/s0167-4781(02)00406-2. [DOI] [PubMed] [Google Scholar]
- Li G, Xiang Y, Sabapathy K, Silverman RH. An apoptotic signaling pathway in the interferon antiviral response mediated by RNase L and c-Jun NH2-terminal kinase. J. Biol. Chem. 2004;279:1123–1131. doi: 10.1074/jbc.M305893200. [DOI] [PubMed] [Google Scholar]
- Li M, Zhou JY, Ge Y, Matherly LH, Wu GS. The phosphatase MKP1 is a transcriptional target of p53 involved in cell cycle regulation. J. Biol. Chem. 2003;278:41059–41068. doi: 10.1074/jbc.M307149200. [DOI] [PubMed] [Google Scholar]
- Li XL, Blackford JA, Hassel BA. RNase L mediates the antiviral effect of interferon through a selective reduction in viral RNA during encephalomyocarditis virus infection. J. Virol. 1998;72:2752–2759. doi: 10.1128/jvi.72.4.2752-2759.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michot B, Hassouna N, Bachellerie JP. Secondary structure of mouse 28S rRNA and general model for the folding of the large rRNA in eukaryotes. Nucleic Acids Res. 1984;12:4259–4279. doi: 10.1093/nar/12.10.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook JL, Leatherman DL. Binding of T-2 toxin to eukaryotic cell ribosomes. Biochem. Pharmacol. 1989a;38:3103–3110. doi: 10.1016/0006-2952(89)90021-x. [DOI] [PubMed] [Google Scholar]
- Middlebrook JL, Leatherman DL. Differential association of T-2 and T-2 tetraol with mammalian cells. J. Pharmacol. Exp. Ther. 1989b;250:860–866. [PubMed] [Google Scholar]
- Moon Y, Pestka JJ. Vomitoxin-induced cyclooxygenase-2 gene expression in macrophages mediated by activation of ERK and p38 but not JNK mitogen-activated protein kinases. Toxicol. Sci. 2002;69:373–382. doi: 10.1093/toxsci/69.2.373. [DOI] [PubMed] [Google Scholar]
- Moon Y, Pestka JJ. Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL-6 expression in mice suppressed by fish oil. J. Nutr. Biochem. 2003;14:717–726. doi: 10.1016/j.jnutbio.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Olivieri F, Prasad V, Valbonesi P, Srivastava S, Ghosal-Chowdhury P, Barbieri L, Bolognesi A, Stirpe F. A systemic antiviral resistance-inducing protein isolated from Clerodendrum inerme Gaertn. is a polynucleotide:adenosine glycosidase (ribosome-inactivating protein) FEBS Lett. 1996;396:132–134. doi: 10.1016/0014-5793(96)01089-7. [DOI] [PubMed] [Google Scholar]
- Olmo N, Turnay J, Gonzalez de BG, Lopez d S I, Gavilanes JG, Lizarbe MA. Cytotoxic mechanism of the ribotoxin alpha-sarcin. Induction of cell death via apoptosis. Eur. J. Biochem. 2001;268:2113–2123. doi: 10.1046/j.1432-1327.2001.02086.x. [DOI] [PubMed] [Google Scholar]
- Pandey M, Bajaj GD, Rath PC. Induction of the interferon-inducible RNA-degrading enzyme, RNase L, by stress-inducing agents in the human cervical carcinoma cells. RNA Biol. 2004;1:21–27. [PubMed] [Google Scholar]
- Park SW, Prithiviraj B, Vepachedu R, Vivanco JM. Isolation and purification of ribosome-inactivating proteins. Methods Mol. Biol. 2006;318:335–347. doi: 10.1385/1-59259-959-1:335. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Smolinski AT. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit Rev. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Zhou HR, Moon Y, Chung YJ. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicol. Lett. 2004;153:61–73. doi: 10.1016/j.toxlet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fonseca C, Amils R, Garrett RA. Fine structure of the peptidyl transferase centre on 23 S-like rRNAs deduced from chemical probing of antibiotic-ribosome complexes. J. Mol. Biol. 1995;247:224–235. doi: 10.1006/jmbi.1994.0135. [DOI] [PubMed] [Google Scholar]
- Scherbik SV, Paranjape JM, Stockman BM, Silverman RH, Brinton MA. RNase L plays a role in the antiviral response to West Nile virus. J. Virol. 2006;80:2987–2999. doi: 10.1128/JVI.80.6.2987-2999.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin VI, Anderson P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J. Biol. Chem. 1999;274:13985–13992. doi: 10.1074/jbc.274.20.13985. [DOI] [PubMed] [Google Scholar]
- Silva AM, Whitmore M, Xu Z, Jiang Z, Li X, Williams BR. Protein kinase R (PKR) interacts with and activates mitogen-activated protein kinase kinase 6 (MKK6) in response to double-stranded RNA stimulation. J. Biol. Chem. 2004;279:37670–37676. doi: 10.1074/jbc.M406554200. [DOI] [PubMed] [Google Scholar]
- Silverman RH, Skehel JJ, James TC, Wreschner DH, Kerr IM. rRNA cleavage as an index of ppp(A2′p)nA activity in interferon-treated encephalomyocarditis virus-infected cells. J. Virol. 1983;46:1051–1055. doi: 10.1128/jvi.46.3.1051-1055.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Tatematsu C, Nakanishi Y. Double-stranded RNA-activated protein kinase interacts with apoptosis signal-regulating kinase 1. Implications for apoptosis signaling pathways. Eur. J. Biochem. 2002;269:6126–6132. doi: 10.1046/j.1432-1033.2002.03325.x. [DOI] [PubMed] [Google Scholar]
- Tumer NE, Hwang DJ, Bonness M. C-terminal deletion mutant of pokeweed antiviral protein inhibits viral infection but does not depurinate host ribosomes. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3866–3871. doi: 10.1073/pnas.94.8.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uptain SM, Kane CM, Chamberlin MJ. Basic mechanisms of transcript elongation and its regulation. Annu. Rev. Biochem. 1997;66:117–172. doi: 10.1146/annurev.biochem.66.1.117. [DOI] [PubMed] [Google Scholar]
- Ushijima H, Rytik PG, Schacke H, Scheffer U, Muller WE, Schroder HC. Mode of action of the anti-AIDS compound poly(I).poly(C12U) (Ampligen): Activator of 2′,5′-oligoadenylate synthetase and double-stranded RNA-dependent kinase. J. Interferon Res. 1993;13:161–171. doi: 10.1089/jir.1993.13.161. [DOI] [PubMed] [Google Scholar]
- Williams BR. Signal integration via PKR. Sci. STKE. 2001;89:RE2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- Witt MF, Pestka JJ. Uptake of the naturally occurring 3-alpha-hydroxy isomer of T-2 toxin by a murine B cell hybridoma. Food Chem. Toxicol. 1990;28:21–28. doi: 10.1016/0278-6915(90)90131-6. [DOI] [PubMed] [Google Scholar]
- Wreschner DH, James TC, Silverman RH, Kerr IM. Ribosomal RNA cleavage, nuclease activation and 2–5A(ppp(A2′p)nA) in interferon-treated cells. Nucleic Acids Res. 1981;9:1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Kumar KU, Kaufman RJ. Identification and requirement of three ribosome binding domains in dsRNA-dependent protein kinase (PKR) Biochemistry. 1998;37:13816–13826. doi: 10.1021/bi981472h. [DOI] [PubMed] [Google Scholar]
- Yang GH, Jarvis BB, Chung YJ, Pestka JJ. Apoptosis induction by the satratoxins and other trichothecene mycotoxins: Relationship to ERK, p38 MAPK, and SAPK/JNK activation. Toxicol. Appl. Pharmacol. 2000;164:149–160. doi: 10.1006/taap.1999.8888. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Islam Z, Pestka JJ. Induction of competing apoptotic and survival signaling pathways in the macrophage by the ribotoxic trichothecene deoxynivalenol. Toxicol. Sci. 2005a;87:113–122. doi: 10.1093/toxsci/kfi234. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Islam Z, Pestka JJ. Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression in spleens of mice exposed to the trichothecene vomitoxin. Toxicol. Sci. 2003a;72:130–142. doi: 10.1093/toxsci/kfg006. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Jia Q, Pestka JJ. Ribotoxic stress response to the trichothecene deoxynivalenol in the macrophage involves the SRC family kinase Hck. Toxicol. Sci. 2005b;85:916–926. doi: 10.1093/toxsci/kfi146. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Lau AS, Pestka JJ. Role of double-stranded RNA-activated protein kinase R (PKR) in deoxynivalenol-induced ribotoxic stress response. Toxicol. Sci. 2003b;74:335–344. doi: 10.1093/toxsci/kfg148. [DOI] [PubMed] [Google Scholar]
- Zhu S, Romano PR, Wek RC. Ribosome targeting of PKR is mediated by two double-stranded RNA-binding domains and facilitates in vivo phosphorylation of eukaryotic initiation factor-2. J. Biol. Chem. 1997;272:14434–14441. doi: 10.1074/jbc.272.22.14434. [DOI] [PubMed] [Google Scholar]