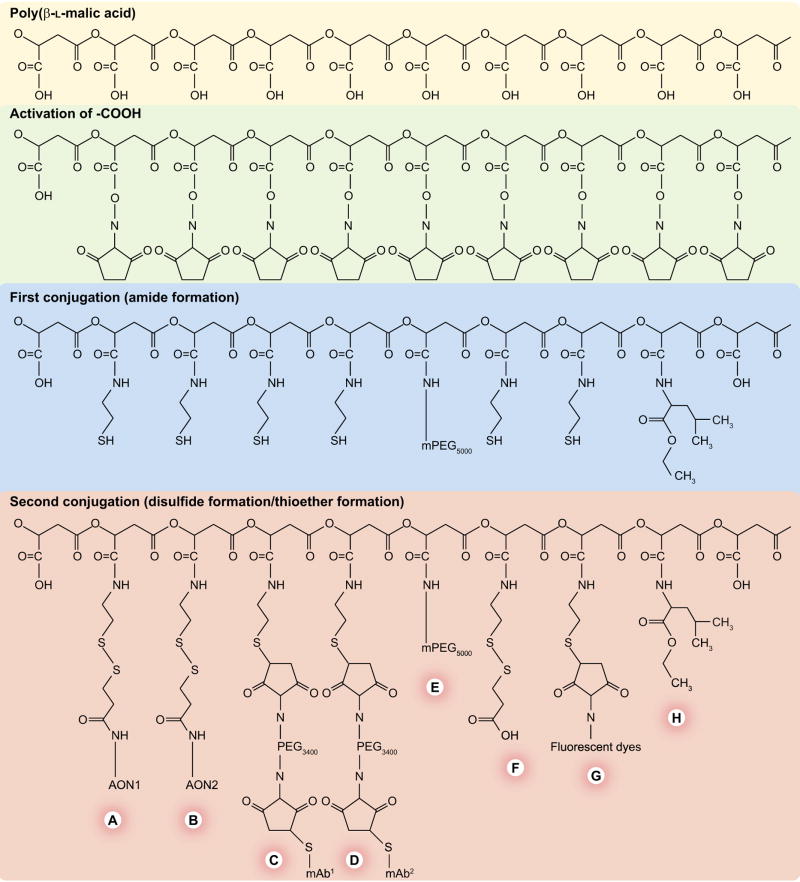
Figure 2. Synthesis of Polycefin nanoconjugate with various functional modules.
Note the hierarchy in the choice of conjugation reactions. The platform consists of biodegradable highly purified β-poly(L-malic acid) from Physarum polycephalum. The carboxyl groups are first activated by esterification with N-hydroxysuccinimide. The hierarchy involves a first series of conjugation reactions with more durable residues, in other words, with polyethylene glycol (PEG), leucine ethylester and the thiol-containing spacer, 2-mercaptoethylamine. In the second series of conjugation reactions, the biochemically fragile molecules, monoclonal antibodies (mAbs) and antisense oligonucleotides (AONs), are conjugated by forming thioether and disulfide bonds, respectively. An optional fluorescent reporter molecule can be similarly conjugated. Nonreacted free thiol groups are then blocked chemically to prevent uncontrolled reactions. The modules are: (A) morpholino AON (to laminin α4 chain) and (B) (to laminin β1 chain) conjugated to the scaffold by disulfide bonds cleavable by the cytoplasmic glutathione to release the free drugs; (C & D) mAbs for cancer cell targeting and receptor-mediated endocytosis; (E) PEG for protection; (F) thiol-masking groups; (G) optional fluorescent reporter dye (fluorescein, Oregon Green or Alexa Fluor 680) for imaging and (H) stretches of conjugated leucine ethyl ester to provide lipophilicity for the disruption of endosomal membranes.