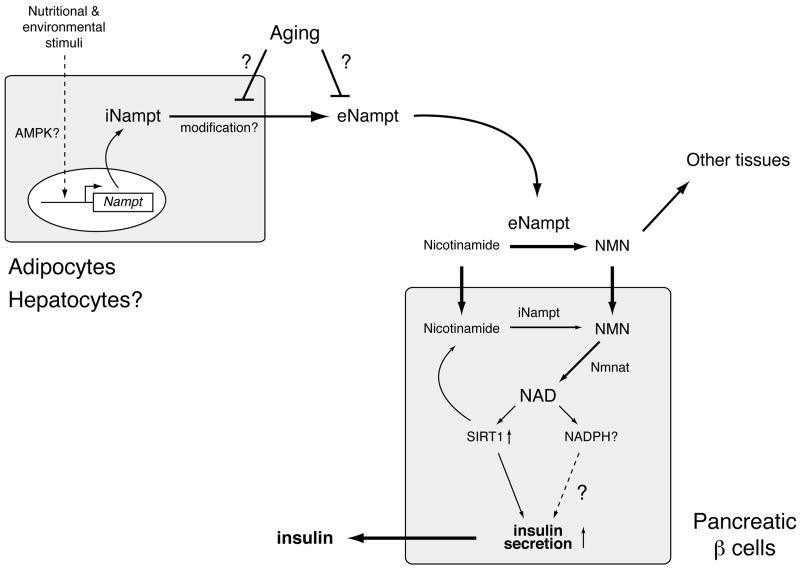
Fig. (2).
A model for the secretion of eNampt and the regulation of glucose-stimulated insulin secretion by Nampt-mediated systemic NAD biosynthesis in pancreatic β cells. Nampt functions as an intra- and extracellular NAD biosynthetic enzyme (iNampt and eNampt). eNampt, which appears to be modified posttranslationally, is positively secreted by fully differentiated adipocytes and possibly by hepatocytes. The expression of the Nampt gene is regulated in response to a variety of nutritional and environmental stimuli, and AMP-activated protein kinase (AMPK) might be involved in this regulation. Nicotinamide, a form of vitamin B3, is converted to nicotinamide mononucleotide (NMN) by iNampt in the cell and also by eNampt in blood circulation. Circulating NMN is distributed to tissues and organs and transported to the inside of cells likely through an unidentified transporter and rapidly converted to NAD by Nmnat. In β cells, Nampt-mediated NAD biosynthesis promotes glucose-stimulated insulin secretion by activating Sirt1 and possibly by increasing other metabolic signals, such as NADPH. Nampt-mediated systemic NAD biosynthesis also declines with advanced age, resulting in reduced Sirt1 activity and insulin secretion in aged β cells. The secretion or the enzymatic activity of eNampt might be affected by aging.