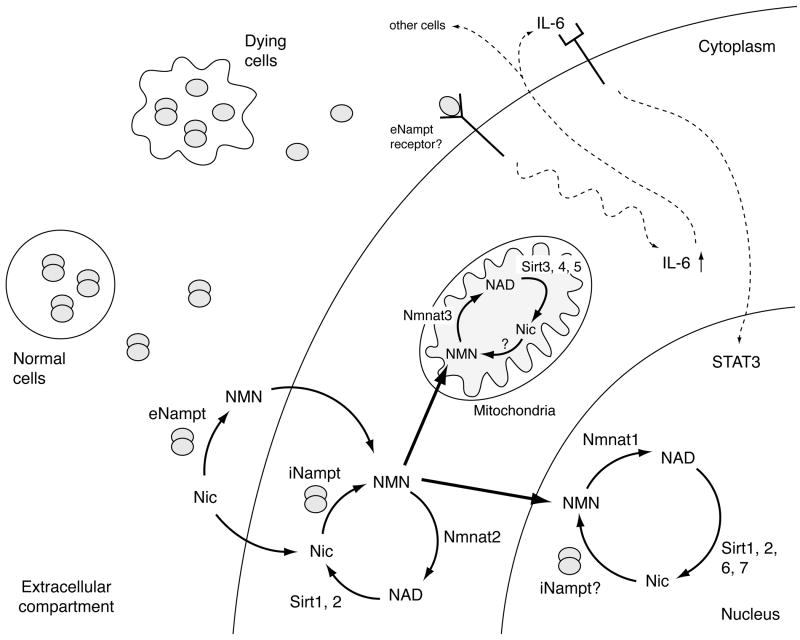
Fig. (3).
The connection between Nampt-mediated NAD biosynthesis and mammalian sirtuins in each subcellular compartment, and a possible dual function of Nampt as an NAD biosynthetic enzyme and a cytokine. There are seven mammalian sirtuin family members, Sirt1-7, and they have distinct subcellular localizations (see Table 1). Although how NAD biosynthesis is regulated in each subcellular compartment is still poorly understood, it is likely that NAD biosynthesis plays a critical role in the regulation of mammalian sirtuin functions in these compartments. As depicted in this scheme, Nampt normally forms a dimer and functions as an NAD biosynthetic enzyme. Nampt produces NMN both intra- and extracellularly, and NMN might also be transported to the nucleus and mitochondria. It is still unclear whether iNampt is directly involved in nuclear and mitochondrial NAD biosynthesis. eNampt has been reported to function as a cytokine, and what regulates this dual role of eNampt is currently unknown. However, based on recent findings, a monomer of Nampt appears to retain a cytokine-like function that stimulates IL-6 production and protects macrophage from cell death. In this scheme, it is speculated that monomers of Nampt might be released from dying cells due to apoptosis or necrosis. See details in texts.