Figure 1.
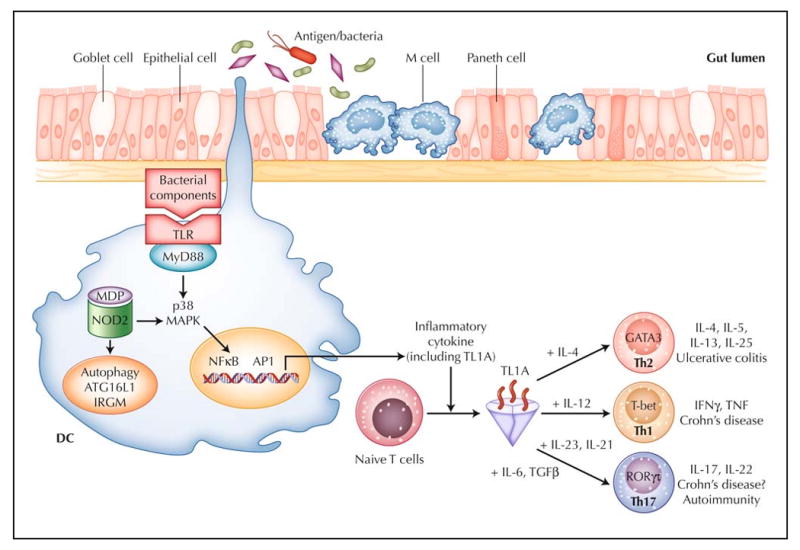
Working hypothesis of inflammatory bowel disease. The intestinal immune system is in close apposition to luminal antigen/bacteria, separated by a single layer of epithelial cells. Goblet cells contribute to the formation of the protective mucus layer, M cells and dendritic cells (DCs) sample intestinal luminal contents. Over-response to antigens, either through the Toll-like receptors (TLR), the intracellular sensor NOD2, or antigen processing via autophagy, results in stimulated DCs that recruit and generate various T-helper-cell subtypes (Th1, Th2, and Th17). TL1A appears to be a critical factor in the generation of Th1, Th2, and Th17 cells. For each T-helper-cell differentiation program, specific transcription factors and cytokine milieu are required. Terminally differentiated T helper cells are characterized by a specific combination of effector cytokines that orchestrate the effector function of the adaptive immune system. Ulcerative colitis appears to be predominately Th2-mediated, whereas Crohn's disease is a predominately Th1- and Th17-mediated process. GATA—GATA binding protein; IFN—interferon; IL—interleukin; IRGM—immunity-related guanosine triphosphatase, M; MAPK—mitogen-activated protein kinase; MDP— muramyl dipeptide; MyD88—myeloid differentiation factor 88; NOD2—nucleotide-binding oligomerization domain containing 2; TGFβ—transforming growth factor β; TNF—tumor necrosis factor; RORγτ—retinoic acid-binding orphan receptor–γτ.
