Abstract
Purpose
Black patients have worse prognoses than whites with breast or colorectal cancer. Mechanisms underlying such disparities have not been fully explored. We examined the role of hospital factors in racial differences in late mortality after surgery for breast or colon cancer.
Methods
Patients undergoing surgery after new diagnosis of breast or colon cancer were identified using the Surveillance Epidemiology and End Results–Medicare linked database (1995 to 2005). The main outcome measure was mortality at 5 years. Proportional hazards models were used to assess relationships between race and late mortality, accounting for patient factors, socioeconomic measures, and hospital factors. Fixed and random effects models were used to account for quality differences across hospitals.
Results
Black patients, compared with white patients, had lower 5-year overall survival rates after surgery for breast (62.1% v 70.4%, respectively; P < .001) and colon cancer (41.3% v 45.4%, respectively; P < .001). After controlling for age, comorbidity, and stage, black race remained an independent predictor of mortality for breast (adjusted hazard ratio [HR] = 1.25; 95% CI, 1.16 to 1.34) and colon cancer (adjusted HR = 1.13; 95% CI, 1.07 to 1.19). After risk adjustment, hospital factors explained 36% and 54% of the excess mortality for black patients with breast cancer and colon cancer, respectively. Hospitals with large minority populations had higher late mortality rates independent of race.
Conclusion
Hospital factors, including quality, are important mediators of the association between race and mortality for breast and colon cancer. Hospital-level quality improvement should be a major component of efforts to reduce disparities in cancer outcomes.
INTRODUCTION
Black patients have substantially lower survival rates compared with whites after an initial diagnosis of breast or colon cancer.1 Such disparities are attributable to a variety of mechanisms. Perhaps reflecting differences in cancer screening and access to care in general, black patients present with later stage cancer and are less likely to undergo cancer-directed surgery than whites.2 Even among patients of similar stage and treatment, racial differences in socioeconomic status (SES), comorbidity rates, and, in some instances, cancer biology could contribute to higher late mortality among black patients.3,4
Although such patient-level factors are no doubt important, racial disparities in surgical outcomes may be as much about the systems in which black patients receive their care. To a large extent, black patients and white patients receive their care at different hospitals5 and by different physicians.6 Relevant to cancer care, several studies have suggested that black patients have less access to high-volume hospitals, subspecialists, and sophisticated diagnostic procedures.7–9 Given the complexity of modern cancer care, it is easy to imagine how such resource disparities could translate into suboptimal processes of care and ultimately into poorer outcomes.
Our previous research, focused on operative mortality, provides direct support for this hypothesis. Black patients had higher mortality largely because they tended to undergo surgery at lower-quality hospitals (ie, at facilities with higher mortality regardless of race).10 In this study, we explored whether hospital factors might similarly explain racial disparities in late mortality after cancer surgery. We focused on breast and colon cancer, two of the most common causes of cancer mortality in the United States, for which disparities in outcome are well documented.
METHODS
Study Population and Data Source
We used files from the 1995 to 2005 Surveillance Epidemiology and End Results (SEER) –Medicare linked database.11,12 The SEER database is a prospective clinical database containing patient demographics and information related to tumor stage, grade, and location and surgical treatment. During the time of this study, the SEER files included 12 registries in five states and seven county-based areas. The Medicare claims data include all billed claims for hospital, outpatient, and physician services, including International Classification of Diseases, 9th revision (ICD-9) diagnostic codes and Current Procedural Terminology (CPT) procedure codes.11
Our study included patients age 65 years and older who underwent breast or colon cancer resection between January 1, 1995 and December 31, 2002, with follow-up until December 31, 2005. Breast cancer patients were identified by an incident diagnosis of breast cancer (SEER cancer site recode 46) and Medicare facility codes indicating surgical resection We used diagnosis codes (174.0 to 175.9), ICD-9 procedure codes (85.12, 85.20 to 85.23, and 85.41 to 85.48), and CPT codes (19120 to 19126, 19180 to 19240, 19160, and 19162) for procedures occurring between 1 month before and 6 months after the month of diagnosis. Colon cancer patients were identified by an incident diagnosis of colon cancer (SEER cancer site recodes 15 to 23 and 25) and Medicare facility codes indicating resection. We used ICD-9 diagnosis codes (153.0 to 154.9), ICD-9 procedure codes (45.41 to 45.49 and 45.70 to 45.80), and CPT codes (44140, 44160, 44202, 44310, and 44320). All patients were enrolled in parts A and B of the fee-for-service Medicare plan for 6 months before and 9 months after diagnosis. This time interval was defined to identify presence of comorbid disease before diagnosis13 and to track provider services (eg, operation) after diagnosis. Exclusion criteria included enrollment in managed care plans, diagnosis of stage 0 or in situ cancer, and cancer diagnosis at autopsy. To minimize statistical artifact as a result of small sample sizes, we excluded patients from hospitals with fewer than 25 breast or colon cancer patients over the 8-year patient ascertainment period. Finally, patients from four SEER sites (Utah, Hawaii, Iowa, and New Mexico) with negligible numbers of black patients were excluded.
Data
Consistent with most previous work on racial disparities, we focused our analyses on black and white race. Patient race was obtained from the Medicare enrollment database, which has at least 95% accuracy in black and white race assignment.14 Age and sex were obtained from the Medicare enrollment database. SES was estimated using median household income at the ZIP code level and 2000 US Census data.15,16 Cancer stage, based on the American Joint Committee on Cancer system,17 was obtained from SEER registry data. Patient comorbidities were assigned using the modified Charlson method.18,19
Analysis
Our primary analyses examined associations between patient race and mortality, both assessed at the patient level. χ2 statistics were used to compare demographic and clinical characteristics of black and white patients. The primary end point was overall survival, which was defined as time from surgery until death from any cause. Analyses using cancer-specific mortality as the primary end point yielded similar results and are not presented herein. Overall survival was censored at 5 years to weight our analysis more toward cancer-related deaths, as opposed to later deaths from other chronic conditions that vary by race.20
Unadjusted analysis of overall survival was performed using Kaplan-Meier estimates, and the log-rank test was used to examine the relationship between race and overall survival. Cox proportional hazards regression was used to assess the effect of race on overall survival, after sequentially adjusting for patient factors, SES, and hospital factors. Patient factors included age, sex, comorbidity, and stage. Because SES reflects aspects of social disadvantage experienced by black patients as a group, this variable was modeled separately from other patient characteristics. In addition, as previously described, area-level SES may be more strongly related to hospital factors than to patient factors associated with surgical risk.21
To account for between-hospital differences, we used three different models. First, we used a model that included hospital volume. Hospital volume was defined by measuring the total number of index colon cancer or breast cancer resections performed in that facility from 1995 through 2002 and then dividing into volume quartiles (very low, low, medium, and high). The analyses were adjusted for clustering within hospitals using robust sandwich estimates for the SE.22 Second, as described in our previous work on operative mortality,10 we used fixed effects models with hospital indicator variables to account for observed and unobserved differences in hospital factors associated with mortality after surgery.23,24 Third, we used a gamma frailty model incorporating hospital-specific random effects25 to capture heterogeneity across hospitals.
To assess the relative contribution of patient and hospital factors to racial differences in mortality in the univariate analysis, we examined the extent to which the unadjusted hazard ratio was attenuated as additional variables were added to the model. The relative attenuation was computed as (HRR – HRROF) ÷ (HRR – 1), where HRR is the (unadjusted) hazard ratio (HR) for 5-year mortality comparing black patients with white patients without consideration of other (patient and hospital) factors, and HRROF is the adjusted HR comparing black patients with white patients after adjustment for each of the other factors (patient, SES, and hospital). In the multivariate analysis, we examined the extent to which sequential adjustment of the model attenuated the HR relative to adjustment for patient factors alone. Although not intended to decompose statistical variance associated with different variables (or categories of variables), this simple measure is nonetheless useful in summarizing the relative contribution of different factors to excess risk of mortality.26 Additionally, we examined the percent increase in generalized R2 as a measure of improvement in model fit.27,28
We performed stratified analyses to further distinguish between within-hospital and between-hospital disparities in mortality. Specifically, as described previously,10 we assessed 5-year mortality according to hospital racial mix (the percentage of black patients among all patients undergoing breast or colon cancer operations at each hospital). Hospitals were grouped into the following quartiles based on hospital racial mix: less than 10% black, 10% to 19%, 20% to 49%, and ≥ 50%. In these stratified analyses, mortality rates in blacks and whites were assessed separately.
P < .05 was considered statistically significant, and all tests were two-sided. All analyses were performed using SAS 9.1 (SAS institute, Cary, NC) and STATA 9.0 (StataCorp, College Station, TX) software. The Institutional Review Board of the University of Michigan approved the study protocol.
RESULTS
The study cohort consisted of 25,571 breast cancer patients and 22,168 colon cancer patients treated in 436 hospitals. Among the cohort, 9.7% of breast cancer patients and 11.8% of colon cancer patients were black. For both breast and colon cancer, black patients presented at younger age with higher tumor stage and lived in areas with lower median household income compared with white patients. Black patients with breast cancer had significantly more comorbidities compared with white patients. A similar trend was observed for colon cancer patients, but the difference was not statistically significant (Table 1).
Table 1.
Patient and Hospital Characteristics Among Patients Who Underwent Breast and Colon Cancer Procedures
| Patient and Hospital Characteristics | % of Patients |
|||||
|---|---|---|---|---|---|---|
| Breast Cancer (n = 25,571) |
Colon Cancer (n = 22,168) |
|||||
| White | Black | P | White | Black | P | |
| Patient population | 90.27 | 9.73 | 88.24 | 11.76 | ||
| Age, years | .0002 | < .0001 | ||||
| 65-69 | 18.74 | 21.51 | 12.37 | 17.65 | ||
| 70-74 | 25.57 | 26.74 | 20.87 | 24.14 | ||
| 75-79 | 25.11 | 24.97 | 24.86 | 24.64 | ||
| 80-84 | 17.9 | 15.24 | 21.51 | 18.15 | ||
| 85+ | 12.68 | 11.54 | 20.39 | 15.43 | ||
| Sex | NA | .0067 | ||||
| Female | 43.78 | 40.98 | ||||
| Male | 56.22 | 59.02 | ||||
| Comorbidity index | < .0001 | .09 | ||||
| 0 | 80.57 | 72.7 | 66.92 | 64.77 | ||
| 1 | 16.57 | 22.52 | 26.99 | 28.86 | ||
| > 2 | 2.87 | 4.78 | 6.1 | 6.37 | ||
| Cancer stage | < .0001 | < .0001 | ||||
| I | 55.11 | 41.21 | 23.47 | 21.11 | ||
| II | 36.98 | 45.03 | 36.88 | 34.54 | ||
| III | 5.63 | 9.81 | 27.15 | 28.2 | ||
| IV | 2.28 | 3.94 | 12.49 | 16.16 | ||
| SES | < .0001 | < .0001 | ||||
| $25,000 or less | 3.3 | 26.66 | 3.89 | 26.75 | ||
| $25,001-$35,000 | 13.43 | 31.32 | 13.54 | 31.89 | ||
| $35,001-$45,000 | 17.72 | 21.43 | 18.12 | 22.03 | ||
| $45,001+ | 65.55 | 20.59 | 64.46 | 19.34 | ||
| Hospital patient volume | < .0001 | < .0001 | ||||
| Very low | 30.74 | 30.84 | 30.97 | 27.36 | ||
| Low | 28.5 | 27.95 | 31.64 | 32.92 | ||
| Median | 19.77 | 14.52 | 19.68 | 14.5 | ||
| High | 21 | 26.7 | 17.71 | 25.21 | ||
| Hospital racial mix | < .0001 | < .0001 | ||||
| < 10% | 74.78 | 22.32 | 74.73 | 20.76 | ||
| 10%-19% | 15.2 | 19.02 | 14.8 | 17.96 | ||
| 20%-49% | 7.68 | 24.65 | 8.73 | 28.59 | ||
| > 50% | 2.34 | 34.02 | 1.74 | 32.69 | ||
Abbreviations: NA, not available; SES, socioeconomic status.
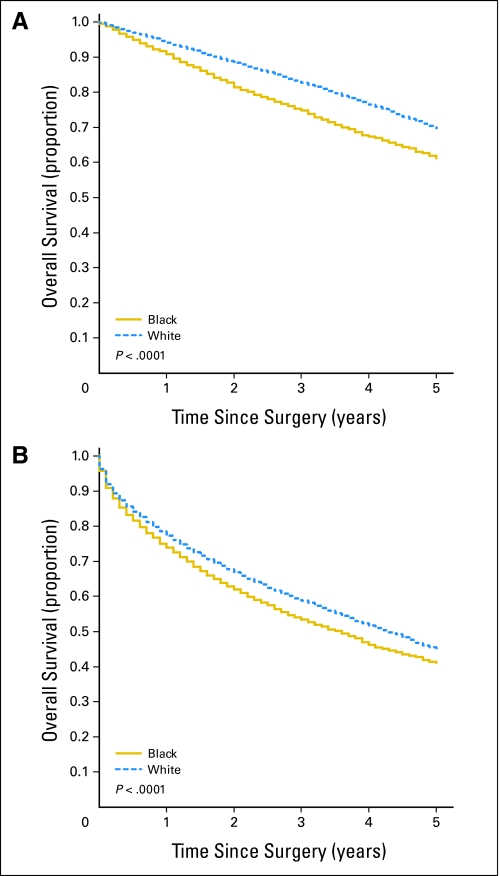
Black patients, compared with white patients, had lower 5-year overall survival rates after surgery for both breast (62.1% v 70.4%, respectively; P < .001) and colon cancer (41.3% v 45.4%, respectively; P < .001; Fig 1). For breast cancer, the unadjusted HR of mortality among black versus white patients was 1.42 (95% CI, 1.32 to 1.52; Table 2). We examined the proportional excess mortality explained individually by patient factors, SES, and hospital factors by comparing HRs when the model was adjusted for each individually with the HR of the unadjusted model. Adjustment for patient factors resulted in 40.5% relative attenuation of the race effect on mortality. In the univariate model, adjustment for the separate effects of SES and the effects of individual hospitals substantially attenuated the HR, whereas adjustment for hospital volume did not.
Fig 1.
Five-year overall survival for black versus white patients with (A) breast cancer and (B) colon cancer.
Table 2.
Influence of Patient and Hospital Variables on 5-Year Overall Survival Among Black and White Patients
| Factor | Breast Cancer |
Colon Cancer |
||||
|---|---|---|---|---|---|---|
| 5-Year Mortality, Black v White |
% of Excess Mortality Explained* | 5-Year Mortality, Black v White |
% of Excess Mortality Explained* | |||
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |||
| Unadjusted | 1.42 | 1.32 to 1.52 | 1.14 | 1.08 to 1.20 | ||
| Adjusted individually for: | ||||||
| Patient factors† | 1.25 | 1.16 to 1.34 | 40.48 | 1.13 | 1.07 to 1.19 | 7.14 |
| SES | 1.28 | 1.18 to 1.38 | 33.33 | 1.08 | 1.02 to 1.14 | 42.86 |
| Hospital volume‡ | 1.44 | 1.34 to 1.55 | NA | 1.16 | 1.10 to 1.23 | NA |
| Hospital fixed effects§ | 1.31 | 1.21 to 1.42 | 26.19 | 1.08 | 1.01 to 1.16 | 42.86 |
| Adjusted sequentially for: | ||||||
| Patient factors | 1.25 | 1.16 to 1.34 | 40.48 | 1.13 | 1.07 to 1.19 | 7.14 |
| Patient factors + SES | 1.16 | 1.08 to 1.26 | 36.00 | 1.08 | 1.02 to 1.14 | 38.46 |
| Patient factors† + hospital fixed effects‡ | 1.16 | 1.07 to 1.26 | 36.00 | 1.06 | 1.00 to 1.14 | 53.84 |
| Patient factors† + hospital fixed effects‡ + SES | 1.13 | 1.03 to 1.23 | 48.00 | 1.03 | 0.96 to 1.11 | 76.92 |
Abbreviations: SES, socioeconomic status; NA, not applicable.
Proportion of excess mortality is relative to unadjusted model for individual factors in the univariate analysis and is relative to the risk-adjusted model (adjusted for patient factors) for the sequential multivariate analysis.
Patient factors include age, sex, comorbidities, and cancer stage.
Hospital volume adjustment also included adjustment for hospital teaching status and cancer center designation, the effects of which were negligible.
Estimates of hazard ratios and 95% CIs were obtained from model including hospital fixed effects.
We examined the proportional excess mortality explained by sequential addition of SES and hospital factors by comparing HRs after each step in the sequential adjustment with the HR after adjusting for only patient factors—that is, after risk adjustment. In the multivariate model, after adjustment for patient factors, further adjustment for SES resulted in 36% attenuation of the HR, as did adjustment for the effects of individual hospitals. Combined adjustment for patient factors, SES, and the effects of individual hospitals resulted in 48% relative attenuation of the HR of mortality among black and white breast cancer patients. The point estimates and the 95% CIs for the adjusted HR obtained from the hospital fixed effects and the random effects models did not differ.
Racial difference in survival among colon cancer patients was not as great as in breast cancer (Table 2). The unadjusted HR of mortality among black versus white patients was 1.14 (95% CI, 1.08 to 1.20). Adjustment for patient factors resulted in 7.1% relative attenuation of the race effect on mortality. Similar to the breast cancer model, individual adjustment for SES and for the effects of individual hospitals substantially attenuated the race effect on mortality, whereas adjustment for hospital volume had no effect. After risk adjustment in the multivariate model, adjustment for SES resulted in 38.5% attenuation of the HR, whereas adjustment for the effects of individual hospitals resulted in 53.8% attenuation. Combined adjustment for patient factors, SES, and the effects of individual hospitals resulted in 76.9% relative attenuation of the HR of mortality among black and white colon cancer patients.
Hospitals treating a higher proportion of black patients had worse 5-year survival for breast and colon cancer among both black and white patients. Overall, patients with breast cancer in hospitals with more than 50% black patients had a 32% increased risk of mortality compared with all patients in a hospital with less than 10% black patients (adjusted HR = 1.32; 95% CI, 1.20 to 1.45). Similarly, all patients with colon cancer in hospitals with more than 50% black patients had a 27% increased risk of mortality compared with all patients in hospitals with less than 10% black patients (adjusted HR = 1.27; 95% CI, 1.18 to 1.37). Within groups of hospitals with similar racial composition, black versus white differences in survival after surgery for breast cancer were considerably smaller than those observed overall (Table 3). For colon cancer, blacks and whites had virtually identical 5-year survival within most of the hospital racial composition strata.
Table 3.
Racial Differences in Late Mortality Among Breast and Colon Cancer Patients Based on Hospital Racial Mix
| Cancer | Hospital Racial Mix* |
|||||||
|---|---|---|---|---|---|---|---|---|
| < 10% |
10%-19% |
20%-49% |
≥ 50% |
|||||
| Adjusted HR of 5-Year Mortality, Black v White† | 95% CI | Adjusted HR of 5-Year Mortality, Black v White | 95% CI | Adjusted HR of 5-Year Mortality, Black v White | 95% CI | Adjusted HR of 5-Year Mortality, Black v White | 95% CI | |
| Breast cancer | 1.05 | 0.89 to 1.23 | 1.23 | 1.03 to 1.47 | 1.19 | 0.99 to 1.44 | 1.13 | 0.89 to 1.44 |
| Colon cancer | 1.03 | 0.91 to 1.67 | 0.99 | 0.85 to 1.15 | 0.99 | 0.86 to 1.14 | 1.11 | 0.90 to 1.37 |
Abbreviation: HR, hazard ratio.
Hospital racial mix represents the percentage of black patients among black and white patients treated in hospital.
Adjusted for patient factors: age, sex (for colon cancer), comorbidities, and cancer stage.
DISCUSSION
Although racial disparities in survival after cancer surgery are no doubt multifactorial, our findings highlight the importance of hospital factors, including quality. After accounting for potentially confounding patient characteristics, black patients had substantially higher late mortality rates after surgery for breast and colon cancer than their white counterparts. For breast cancer, patient factors played a greater proportional role than hospital factors in explaining the survival disparity observed for black patients. For colon cancer, hospital factors accounted for nearly half of the excess late mortality risk, whereas patient factors explained relatively little of the disparity. Hospitals with large minority populations had significantly higher late mortality rates for both cancers among white and black patients.
Our study is not the first to demonstrate the importance of system factors in explaining racial disparities in outcomes with specific conditions or procedures. In addition to our previous study focusing on operative mortality with different procedures,10 other investigators have observed lower rates of referral to high-volume centers for minority patients when compared with whites8 and clustering of racial and ethnic minorities in a small number of centers.5 In addition, black patients have less access to high-quality surgeons,29 are more likely to experience treatment delays,2,30 and may be less likely to receive adjuvant therapy.31,32
Although our analysis demonstrates the importance of hospital factors in explaining racial disparities in late mortality after cancer surgery, it does not identify specifically what those factors are. Among potential candidates, patients cared for in poorer hospitals with fewer resources may have reduced access to processes of care such as multidisciplinary management teams and high-quality imaging technology and may be less likely to receive evidence-based adjuvant therapy after surgical resection.32–36 Such hospitals may tend to allocate their resources more toward clinical conditions most prevalent in disadvantaged groups, including trauma and emergency care and infectious diseases.
This study has several limitations. First, we studied only Medicare patients greater than 65 years of age. Although we have no reason to believe that hospital factors would be less important in mediating outcomes among younger patients, our analysis could not confirm this empirically. Our reliance on patients with Medicare insurance is a more important limitation, however. Racial disparities in late survival after cancer care may be even more pronounced in the large proportion of minority patients without insurance. Such patients may present with poorly managed comorbidities and later stage cancers (as a result of lack of screening). They may also tend to receive their care in resource-poor safety net hospitals. For these reasons, addressing systems problems and quality may be particularly important in reducing disparities in younger, uninsured populations.
Second, because we relied on administrative data to identify patient comorbidities, we may have underestimated their contribution to mortality after cancer surgery. Previous studies have documented the importance of coexisting noncancer diagnoses on prognosis.20,37–39 In additional analyses (not shown), blacks and whites had identical other-cause mortality for colon cancer (22% v 22%, respectively, at 5 years) and similar rates for breast cancer (18% v 15%, respectively). Instead, racial differences in overall mortality rates were almost entirely a result of differences in cancer-specific mortality. These data support our main conclusions that racial disparities in survival after cancer surgery relate primarily to differences in cancer care, not comorbidity prevalence or management.
A third, important limitation of this study is our lack of patient-level measures of income, education, and other socioeconomic variables associated with both race and mortality. In our analysis, area-level SES was an important mediator of relationships between race, hospital factors, and late mortality. These findings are consistent with previous research indicating that hospitals serving patients residing in areas with low SES have worse surgical outcomes independent of race.21 Although previous studies suggest that area-level SES data correlate strongly with patient-level data,40 individual-level data would be nonetheless invaluable for fully exploring the role of SES in explaining both between-hospital and within-hospital differences in survival by race.
Fourth, in our analyses of hospital racial mix and mortality, we had limited statistical power for examining potential important hospital subgroups. Only a small proportion of hospitals (11.87% in this study) served at least 20% black patients. In only 4.79% of hospitals did blacks constitute the majority. Furthermore, our study was limited to the one state and seven SEER counties with sufficiently large minority representation, further limiting the generalizability of our findings. Finally, we focused primarily on mortality in black patients versus white patients. Future studies might consider the extent to which hospital factors may be important in outcomes after cancer surgery for other racial and ethnic groups.
Understanding and ultimately reducing racial disparities in health care has become an important research and policy priority. With regard to cancer care, broad efforts aimed at both improving cancer prevention and early detection with screening are no doubt essential. However, our findings suggest that where patients obtain treatment after cancer diagnosis may be equally important. Among potential approaches to reducing hospital-related disparities, payers and policy makers could aim to direct black patients and other disadvantaged groups to hospitals and systems with better results in cancer care—so-called selective referral. Although more expedient, such strategies are currently limited by reliable data for identifying best hospitals. As implied by our results, simple measures, such as hospital procedure volume, would not be sufficient for steering black patients to centers with better outcomes and eliminating disparities. Moreover, strategies focused exclusively on selective referral might disrupt coordination of care for minority patients and have other unintended harms.
For these reasons, improving quality in the systems in which patients currently receive their cancer care is paramount. Effecting this goal will require a better understanding of structural differences and resource deficits that account for worse cancer outcomes at hospitals treating larger minority populations. A better understanding of differences in process of care is also essential. Further research in this area should aim to understand which aspects of standard treatment are poorly delivered or absent within hospitals that disproportionately serve minority patients. Perhaps more importantly, researchers should delineate better the obstacles to cancer treatment in these hospitals. Overcoming such obstacles will likely require special efforts to coordinate and deliver care, such as directed patient navigator programs and targeted community outreach. To be consistent with the goals of improving equity and quality in cancer care, these efforts should extend well beyond the current policy-based impetus to reward or penalize hospitals according to standard measures of cancer care quality.
Footnotes
Supported by Mentored Research Scholar Grant No. MRSGT06-076-01-CHPHS (A.M.M.) from the American Cancer Society, Atlanta, GA, and by Senior Scientist Award No. K05 CA115571-01 (J.D.B.) from the National Cancer Institute.
The views expressed herein do not necessarily represent the views of the American Cancer Society, the National Cancer Institute, Center for Medicare and Medicaid Services, or the US Government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Arden M. Morris, Sandra L. Wong, Emily V. Finlayson, John D. Birkmeyer
Collection and assembly of data: Arden M. Morris, Niya Gu
Data analysis and interpretation: Tara M. Breslin, Arden M. Morris, Niya Gu, Sandra L. Wong, Emily V. Finlayson, Mousumi Banerjee, John D. Birkmeyer
Manuscript writing: Tara M. Breslin, Arden M. Morris, Mousumi Banerjee, John D. Birkmeyer
Final approval of manuscript: Tara M. Breslin, Arden M. Morris, Sandra L. Wong, Emily V. Finlayson, Mousumi Banerjee, John D. Birkmeyer
REFERENCES
- 1.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 2.Lund MJ, Brawley OP, Ward KC, et al. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109:545–557. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 3.Newman LA, Griffith KA, Jatoi I, et al. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham JE, Butler WM. Racial disparities in female breast cancer in South Carolina: Clinical evidence for a biological basis. Breast Cancer Res Treat. 2004;88:161–176. doi: 10.1007/s10549-004-0592-9. [DOI] [PubMed] [Google Scholar]
- 5.Schatzkin A. Variation in inpatient racial composition among acute-care hospitals in New York State. Soc Sci Med. 1985;20:371–379. doi: 10.1016/0277-9536(85)90012-7. [DOI] [PubMed] [Google Scholar]
- 6.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 8.Liu JH, Zingmond DS, McGory ML, et al. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296:1973–1980. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- 9.Lathan CS, Neville BA, Earle CC. Racial composition of hospitals: Effects on surgery for early-stage non–small-cell lung cancer. J Clin Oncol. 2008;26:4347–4352. doi: 10.1200/JCO.2007.15.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas FL, Stukel TA, Morris AM, et al. Race and surgical mortality in the United States. Ann Surg. 2006;243:281–286. doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.Finlayson EV, Birkmeyer JD, Stukel TA, et al. Adjusting surgical mortality rates for patient comorbidities: More harm than good? Surgery. 2002;132:787–794. doi: 10.1067/msy.2002.126509. [DOI] [PubMed] [Google Scholar]
- 14.Arday SL, Arday DR, Monroe S, et al. HCFA's racial and ethnic data: Current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 15.Gornick ME, Eggers PW, Reilly TW, et al. Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med. 1996;335:791–799. doi: 10.1056/NEJM199609123351106. [DOI] [PubMed] [Google Scholar]
- 16.Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene FL, Page DL, Balch CM, et al. AJCC Cancer Staging Handbook. ed 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 18.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Tammemagi CM, Nerenz D, Neslund-Dudas C, et al. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 21.Birkmeyer NJ, Gu N, Baser O, et al. Socioeconomic status and surgical mortality in the elderly. Med Care. 2008;46:893–899. doi: 10.1097/MLR.0b013e31817925b0. [DOI] [PubMed] [Google Scholar]
- 22.Lin D, Wei L. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 23.Localio AR, Berlin JA, Ten Have TR, et al. Adjustments for center in multicenter studies: An overview. Ann Intern Med. 2001;135:112–123. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 24.Berlin JA, Kimmel SE, Ten Have TR, et al. An empirical comparison of several clustered data approaches under confounding due to cluster effects in the analysis of complications of coronary angioplasty. Biometrics. 1999;55:470–476. doi: 10.1111/j.0006-341x.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- 25.Therneau TM, Grambsch PM. Modeling Survival Data Extending the Cox Model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 26.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 27.Schemper M, Stare J. Explained variation in survival analysis. Stat Med. 1996;15:1999–2012. doi: 10.1002/(SICI)1097-0258(19961015)15:19<1999::AID-SIM353>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Allison P. Survival Analysis Using the SAS System. Cary, NC: SAS Institute; 2004. [Google Scholar]
- 29.Mukamel DB, Murthy AS, Weimer DL. Racial differences in access to high-quality cardiac surgeons. Am J Public Health. 2000;90:1774–1777. doi: 10.2105/ajph.90.11.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 31.Foley KL, Kimmick G, Camacho F, et al. Survival disadvantage among Medicaid-insured breast cancer patients treated with breast conserving surgery without radiation therapy. Breast Cancer Res Treat. 2007;101:207–214. doi: 10.1007/s10549-006-9280-2. [DOI] [PubMed] [Google Scholar]
- 32.Morris AM, Billingsley KG, Hayanga AJ, et al. Residual treatment disparities after oncology referral for rectal cancer. J Natl Cancer Inst. 2008;100:738–744. doi: 10.1093/jnci/djn396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elmore JG, Nakano CY, Linden HM, et al. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care. 2005;43:141–148. doi: 10.1097/00005650-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 35.Baldwin LM, Dobie SA, Billingsley K, et al. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97:1211–1220. doi: 10.1093/jnci/dji241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris AM, Wei Y, Birkmeyer NJ, et al. Racial disparities in late survival after rectal cancer surgery. J Am Coll Surg. 2006;203:787–794. doi: 10.1016/j.jamcollsurg.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Morris AM, Baldwin LM, Matthews B, et al. Reoperation as a quality indicator in colorectal surgery: A population-based analysis. Ann Surg. 2007;245:73–79. doi: 10.1097/01.sla.0000231797.37743.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemmens VE, Janssen-Heijnen ML, Houterman S, et al. Which comorbid conditions predict complications after surgery for colorectal cancer? World J Surg. 2007;31:192–199. doi: 10.1007/s00268-005-0711-8. [DOI] [PubMed] [Google Scholar]
- 39.Janssen-Heijnen ML, Maas HA, Houterman S, et al. Comorbidity in older surgical cancer patients: Influence on patient care and outcome. Eur J Cancer. 2007;43:2179–2193. doi: 10.1016/j.ejca.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Bach PB, Schrag D, Brawley OW, et al. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]