Abstract
The dioxygenation of nitric oxide by oxyheme in globin proteins is a major route for NO detoxification in aerobic biological systems. In myoglobin, this reaction is thought to proceed through an iron(III)-bound peroxynitrite before homolytic cleavage of the O-O bond to form an iron(IV)-oxo and NO2 radical followed by recombination and nitrate production. Single turnover experiments at alkaline pH have revealed the presence of a millisecond high-spin heme intermediate. It is widely presumed that this species is an iron(III)-peroxynitrite species, but detailed characterization of the intermediate is lacking. Using resonance Raman spectroscopy and rapid-freeze quench techniques, we identify the millisecond intermediate as an iron(III)-nitrato complex with a symmetric NO2 stretch at 1282 cm−1. Greater time resolution techniques will be required to detect the putative iron(III) peroxynitrite complex.
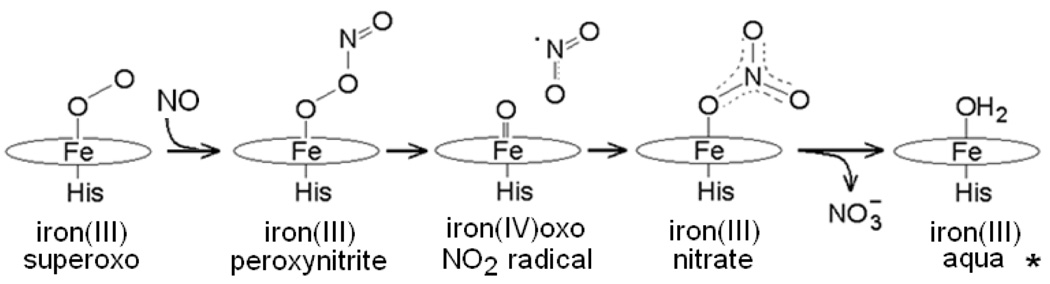
Nitric oxide (NO) is an important signaling molecule in mammals, influencing such diverse functions as immune response, vasodilation, and smooth muscle contraction among others.1 While submicromolar NO concentrations are sufficient to perform these signaling functions, higher concentrations are toxic, via inhibition of essential enzymes including metalloenzymes from the respiratory chain.2 In aerobic environments, NO's cytotoxicity is associated with its near diffusion-limited reaction rate with superoxide to generate peroxynitrite.3 Myoglobin and hemoglobin have long been recognized for their role in O2 storage and delivery, respectively. More recently, oxymyoglobin (Mb-O2) and oxyhemoglobin have been shown to be potent sinks for NO in vivo.4, 5 Mb-O2 converts NO to nitrate (NO3−) with complete retention of the isotopic constitution of the O2 and NO reactants. The mechanism is presumed to proceed via a high-spin ferric peroxynitrite intermediate before homolytic cleavage of the O-O bond and recombination of the NO2 radical with the ferryl-oxo to form the nitrate product (Figure 1). The same reaction mechanism is likely to be the source of the NO dioxygenase activity reported in bacterial flavohemoglobins.5, 6
Figure 1.
Proposed NO dioxygenation by oxyheme (* at alkaline pH, the iron(III)aqua complex of Myoglobin is replaced by an iron(III)hydroxo complex, which adopts a low-spin configuration at cryogenic temperatures).
There are no observable intermediates in this reaction at neutral pH, but stopped-flow UV-vis experiments at alkaline pH show the rapid buildup and decay of a high-spin ferric species.7, 8 Rapid-freeze quench (RFQ) samples of the reaction analyzed by EPR spectroscopy also support the formation of a ferric high-spin transient species.9 For myoglobin at pH 9.5 and 20° C, stopped-flow results indicate that the build-up in high-spin intermediate is maximal within the dead-time of the instrument (1 to 3 ms) and decays fully within 15 to 20 ms. On the basis of the UV-vis characteristics of the intermediate, the species was assigned to an iron(III)-peroxynitrite complex.7, 8 Here, we report the first characterization of this millisecond intermediate by resonance Raman spectroscopy and identify this species as an iron(III)-nitrato complex rather than an iron(III)-peroxynitrite complex.
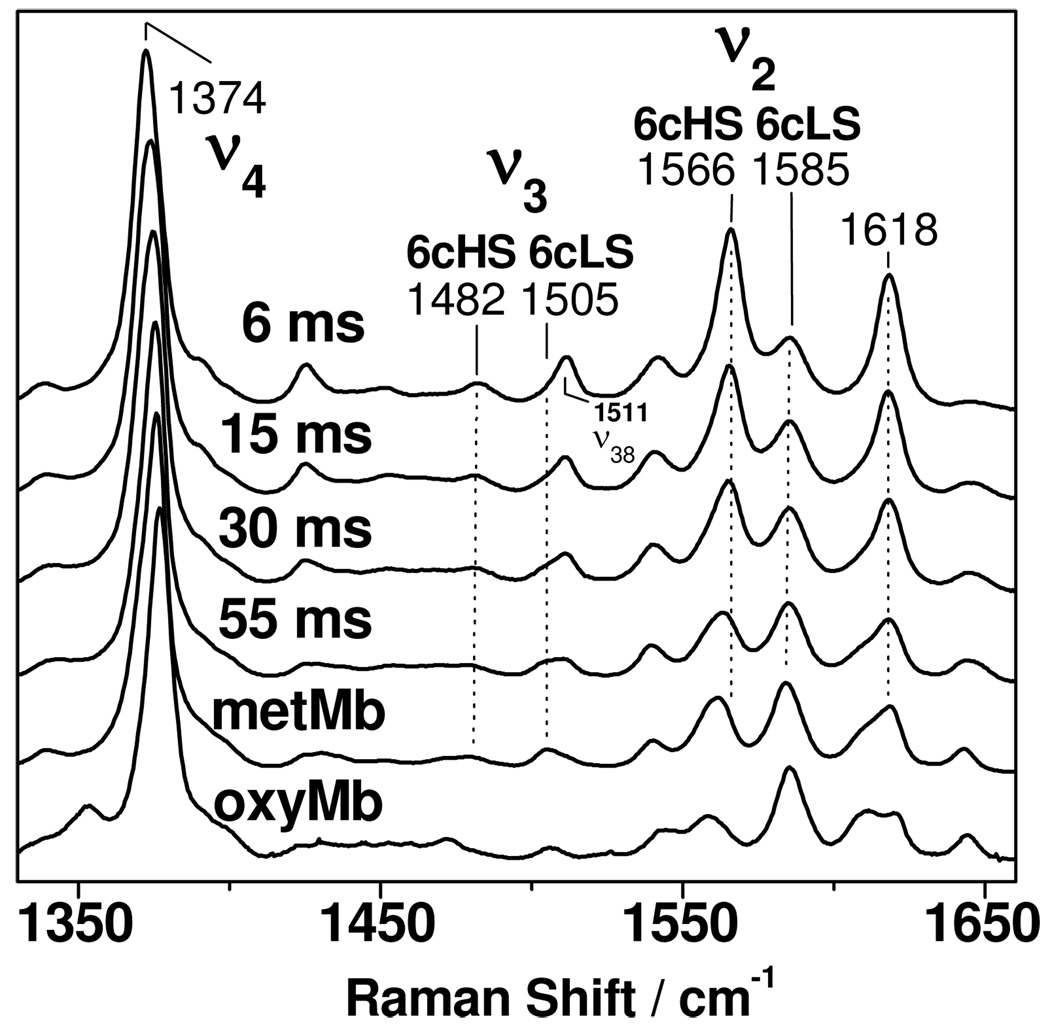
Resonance Raman (RR) spectra obtained with Soret excitation are highly sensitive to the oxidation, spin, and coordination states of the heme iron.10 This technique is an ideal analytical tool for studying the NO dioxygenation reaction since both starting Mb-O2 and final met-Mb are six-coordinate low-spin species at low temperature and alkaline pH, while the putative intermediate is a six-coordinate high-spin species. Figure 2 shows the high-frequency RR spectra of RFQ samples taken at different time points during the reaction of Mb-O2 with NO at pH 9.5 and 3° C. The RFQ sample with the shortest reaction time is trapped within 6 ms (see Supporting Information for experimental details). Its RR high-frequency spectrum exhibits strong ν2 and ν3 porphyrin skeletal modes at 1566 and 1482 cm−1, characteristic of six-coordinate high-spin vibrations, while their low-spin counterparts11 at 1585 and 1505 cm−1 are weak. In contrast, the RR spectra of RFQ samples with longer reaction times show weak high-spin features, and the gradual conversion of high-spin to low-spin species is essentially complete by 55 ms (Figure 2).
Figure 2.
High-frequency RR spectra obtained with 413-nm excitation at 105 K on RFQ samples (reaction times: 6 ms, 15 ms, 30 ms, and 55 ms) of Mb-O2 + NO at pH 9.5. Also shown are the RR spectra of the product and starting material at pH 9.5, metMb and oxyMb, respectively.
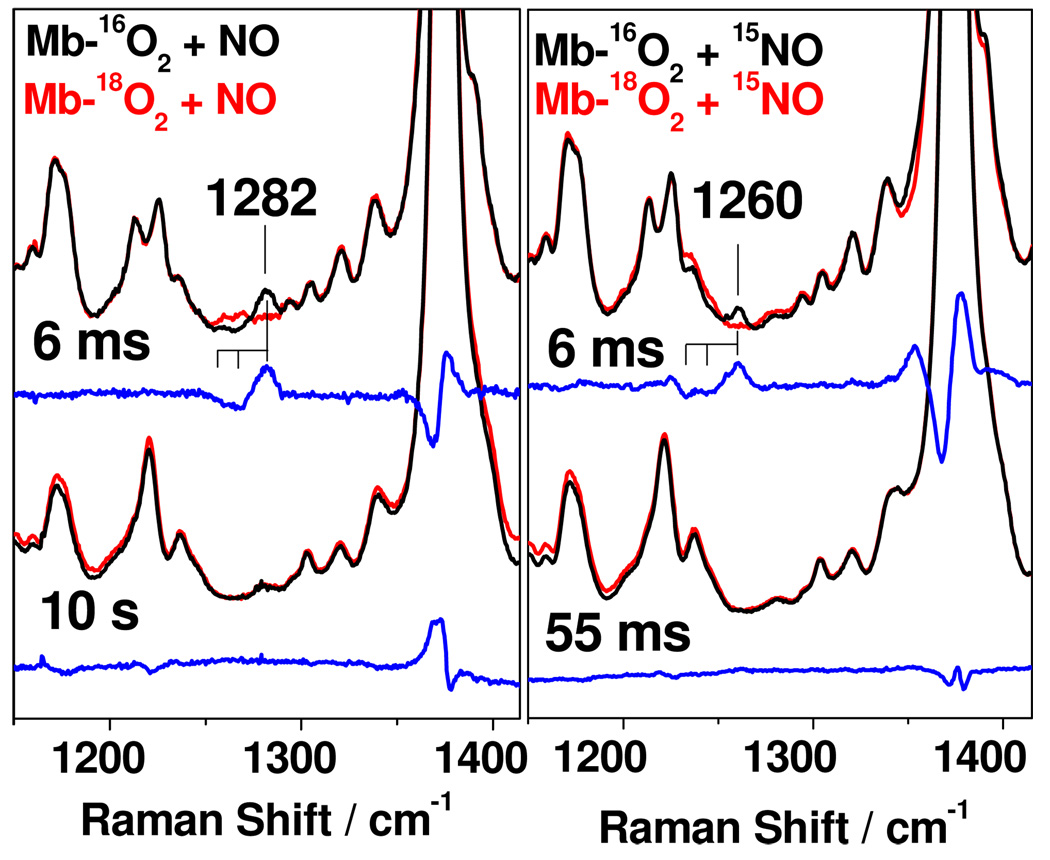
RFQ samples of the reaction of Mb-16O2 and Mb-18O2 with 14NO, 15NO, or 15N18O were used to isolate vibrational modes involving the exogenous ligand. RR spectra of the 6-ms RFQ samples of these reactions reveal a mode at 1282 cm−1 that downshifts with Mb-18O2 and is observed at 1260 cm−1 in Mb-16O2 + 15NO (Figure 3). This RR band is not observed in the RR spectra of samples trapped after longer reaction times, and accordingly, it is assigned to a ligand vibration from the high-spin ferric millisecond intermediate species (Figure 3). As expected, the low-frequency RR spectra of Mb-O2 exhibit a ν(Fe-O2) at 577 cm−1 that shifts −26 cm−1 with 18O2, but no isotope sensitive modes could be detected in the low-frequency RR spectra of the 6-ms RFQ samples (Figure S1). Additional testing of these samples with 351- and 458-nm laser excitations on either side of the Soret absorption did not enhance any new isotope sensitive vibrations (data not shown).
Figure 3.
Mid-frequency RR spectra of RFQ samples for the reaction of 14NO (left) and 15NO (right) with Mb-16O2 (black) versus Mb-18O2 (red). Difference spectra are shown in blue; the differential signals occurring at the intense ν4 mode (1374 cm−1) vary in independent experiments and represent less than 10% of the integrated ν4 area.
The frequency and isotope sensitivity of the 1282-cm−1 signal are not readily matched with expectations for peroxynitrite vibrations. 12 Instead, the 1282-cm−1 Raman band is consistent with a νs(NO2) from an iron(III)-nitrato complex. For example, the six-coordinate high-spin Fe(TPP)(η1 -ONO2)(THF) complex shows FTIR spectra with a strong νs(NO2) at 1280 cm−1 that shifts −22 cm−1 with 15N.13 The assignment of the 1282-cm−1 signal to a nitrato νs(NO2) in the RR spectra of the 6-ms intermediate is further supported by the impact of 18O-labeling of Mb-O2 and NO. Indeed, labeling of all three O-atoms produces the largest downshift of this mode to 1232 cm−1 (Figure S2 shows the RR spectrum of Mb-18O2 + 15N18O). In contrast, when Mb-18O2 is reacted with N16O, two νs(NO2) frequencies are observed (Figure 3): the band with the largest downshift corresponds to Fe-16ON18O2 and the other to Fe-18ON(16O)18O. While the FTIR spectra of Fe(TPP)(η1-ONO2)(THF) show a weak νas(NO2) at 1491 cm−1 and a very weak ν(N-O) near 1000 cm−1, 13 these same modes are also expected to be weak in RR, and it is not surprising that they are not observed in the RR spectra of the 6-ms RFQ samples.
Although our results do not support the proposed peroxynitrite assignment by Herold and coworkers,8 the trapping of an iron(III)-nitrato complex is not inconsistent with a recent theoretical study by Blomberg and coworkers.14 Using density functional theory (DFT), the decay of the peroxynitrite transient via homolytic cleavage of the O-O bond was predicted to occur with an energy barrier insufficient for accumulation of this species in the course of the reaction. Moreover, attempts to model the stabilization of a high-spin millisecond intermediate at alkaline pH as a peroxynitrite, via distal or proximal perturbations, were unsuccessful.14 In contrast, because anionic ligands show decreasing dissociation rate constants with increasing pH,15 stabilization of the iron(III)-nitrato complex is expected at alkaline pH, and is supported by our results. The assignment of the ms-intermediate to an iron(III)-nitrato complex was rejected by Herold and coworkers on the basis of differences between the absorption spectrum of the ms-intermediate and resting Mb with nitrate.8 However, a very large excess of nitrate (ca. 105 equiv) is required to affect the UV-vis spectrum of metMb and it is unclear whether the spectral changes reflect coordination of nitrate to the heme iron(III), or less specific conformational changes. Control RFQ-RR experiments with metMb and high concentrations of nitrite or nitrate at pH 9.5 did not reveal formation of any high-spin heme species or vibrations involving exogenous ligands.
In conclusion, our RR analysis of RFQ samples of the reaction of oxy-Mb + NO at pH 9.5 identifies the ms-intermediate as an iron(III)-nitrato complex. It is important to stress that this species is not observed under equilibrium conditions, even with a high excess of nitrate, and thus corresponds to a transient iron(III) complex. Assigning the ms-intermediate species to a nitrate rather than a peroxynitrite complex resolves the discrepancy between theoretical and experimental studies of the NO dioxygenation reaction in Mb. Our results do not invalidate the proposed reaction mechanism shown in Figure 1. Rather, they indicate that a greater time resolution will be required to determine whether a heme-peroxynitrite intermediate forms in the course of this reaction, and to structurally characterize this species. Such experiments with a microsecond freeze-quench apparatus16 are underway.
Supplementary Material
Experimental details, low-frequency RR spectra of the Mb-O2 + NO reaction, and mid-frequency RR spectra of the reaction with 15N18O. This material is available free of charge via internet at http://pubs.acs.org.
Acknowledgment
This work was supported by the NIH (P.M.-L. GM074785).
References
- 1.(a) Moncada S, Palmer RM, Higgs EA. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]; (b) Lancaster J., Jr . Nitric oxide: Principles and actions. San Diego: Academic press, inc.; 1996. [Google Scholar]; (c) Fukuto JM, Wink DA. Met. Ions Biol. Syst. 1999;36:547–595. [PubMed] [Google Scholar]
- 2.(a) Fang FC. J. Clin. Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nathan C, Shiloh MU. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8841–88418. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gardner PR, Martin LA, Hall D, Gardner AM. Free Radic. Biol. Med. 2001;31:191–204. doi: 10.1016/s0891-5849(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 3.(a) Huie RE, Padmaja S. Free Radic. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]; (b) Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kissner R, Nauser T, Bugnon P, Lye PG, Koppenol WH. Chem. Res. Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Poole RK, Hughes MN. Mol. Microbiol. 2000;36:775–783. doi: 10.1046/j.1365-2958.2000.01889.x. [DOI] [PubMed] [Google Scholar]; (b) Brunori M. Trends Biochem. Sci. 2001;26:209–210. doi: 10.1016/s0968-0004(01)01824-2. [DOI] [PubMed] [Google Scholar]
- 5.Gardner PR. J. Inorg. Biochem. 2005;99:247–266. doi: 10.1016/j.jinorgbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.(a) Gardner PR, Gardner AM, Martin LA, Salzman AL. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gardner AM, Gardner PR. J. Biol. Chem. 2002;277:8166–8171. doi: 10.1074/jbc.M110470200. [DOI] [PubMed] [Google Scholar]
- 7.Herold S. FEBS Lett. 1999;443:81–84. doi: 10.1016/s0014-5793(98)81345-8. [DOI] [PubMed] [Google Scholar]
- 8.Herold S, Exner M, Nauser T. Biochemistry. 2001;40:3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 9.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. Free Radic. Biol. Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Spiro TG. Resonance Raman Spectroscopy of Metalloporphyrins. New York: John Wiley & Sons; 1988. [Google Scholar]
- 11.Feis A, Marzocchi MP, Paoli M, Smulevich G. Biochemistry. 1994;33:4577–4583. doi: 10.1021/bi00181a019. [DOI] [PubMed] [Google Scholar]
- 12.(a) Tsai J-HM, Harrison JG, Martin JC, Hamilton TP, van der Woerd M, Jablonsky MJ, Beckman JS. J. Am. Chem. Soc. 1994;116:4115–4116. [Google Scholar]; (b) Lo W-J, Lee Y-P, Tsai J-HM, Tsai H-H, Hamilton TP, Harrison JG, Beckman JS. J. Chem. Phys. 1995;103:4026–44034. [Google Scholar]
- 13.Gulyan GM, Kurtikyan TS, Ford PC. Inorg. Chem. 2008;47:787–789. doi: 10.1021/ic702102j. [DOI] [PubMed] [Google Scholar]
- 14.Blomberg LM, Blomberg MRA, Siegbahn PEM. J. Biol. Inorg. Chem. 2004;9:923–935. doi: 10.1007/s00775-004-0585-5. [DOI] [PubMed] [Google Scholar]
- 15.(a) Merryweather J, Summers F, Vitello LB, Erman JE. Arch. Biochem. Biophys. 1998;358:359–368. doi: 10.1006/abbi.1998.0872. [DOI] [PubMed] [Google Scholar]; (b) Wanat A, Gdula-Argasinska J, Rutkowska-Zbik D, Witko M, Stochel G, van Eldik R. J. Biol. Inorg. Chem. 2002;7:165–176. doi: 10.1007/s007750100284. [DOI] [PubMed] [Google Scholar]
- 16.(a) Cherpanov AV, de Vries S. Biochim. Biophys. Acta. 2004;1656:1–31. doi: 10.1016/j.bbabio.2004.02.006. [DOI] [PubMed] [Google Scholar]; (b) Wiertz FGM, Richter O-MH, Cherepanov AV, MacMillan F, Ludwig B, de Vries S. FEBS Lett. 2004;575:127–130. doi: 10.1016/j.febslet.2004.08.048. [DOI] [PubMed] [Google Scholar]; (c) Lu S, Wiertz FGM, de Vries S, Moënne-Loccoz P. J. Raman Spectrosc. 2005;36:359–362. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details, low-frequency RR spectra of the Mb-O2 + NO reaction, and mid-frequency RR spectra of the reaction with 15N18O. This material is available free of charge via internet at http://pubs.acs.org.