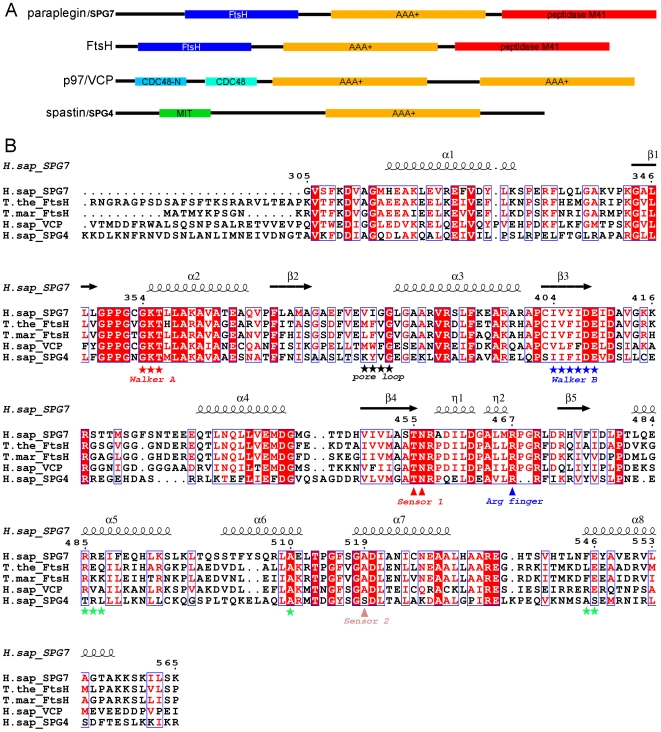
Figure 1. Domain arrangement and sequence comparison of paraplegin/SPG7 and related AAA+ proteins.
A. Domain arrangement of paraplegin, FtsH, VCP, and spastin. Homologies included are the FtsH extracellular (Pfam entry PF36480), AAA+ (PF00004), metallopeptidase M41 (PF01434), Cell division protein-48 (CDC48) N-terminal (PF02359), CDC48-2 (PF02933), and microtubule interacting and transport (MIT; PF04212) domains. B. Sequence alignment of the ATPase domains of paraplegin and related proteins to illustrate the positions of conserved residues. Residue numbering and secondary structural elements are indicated for paraplegin (PDB entry 2qz4) above the alignment. Walker A and B, and Sensor 1 and 2 motifs, the arginine residue predicted to act as an arginine finger, as well as the pore loop are indicated below the alignment. Indicated by green asterisks are HSP disease related positions. Sequences shown are human paraplegin/SPG7 (residues 305–565; PDB entry 2qz4; gene identification code 116242796). Thermus thermophilus FtsH (126–624; 2dhr; gi:8051696), Thermotoga maritima FtsH (147–610; 2cea; gi:15643346), human p97/VCP (116–417; gi:112818458), and human spastin/SPG4 (114–437; gi:11875211).