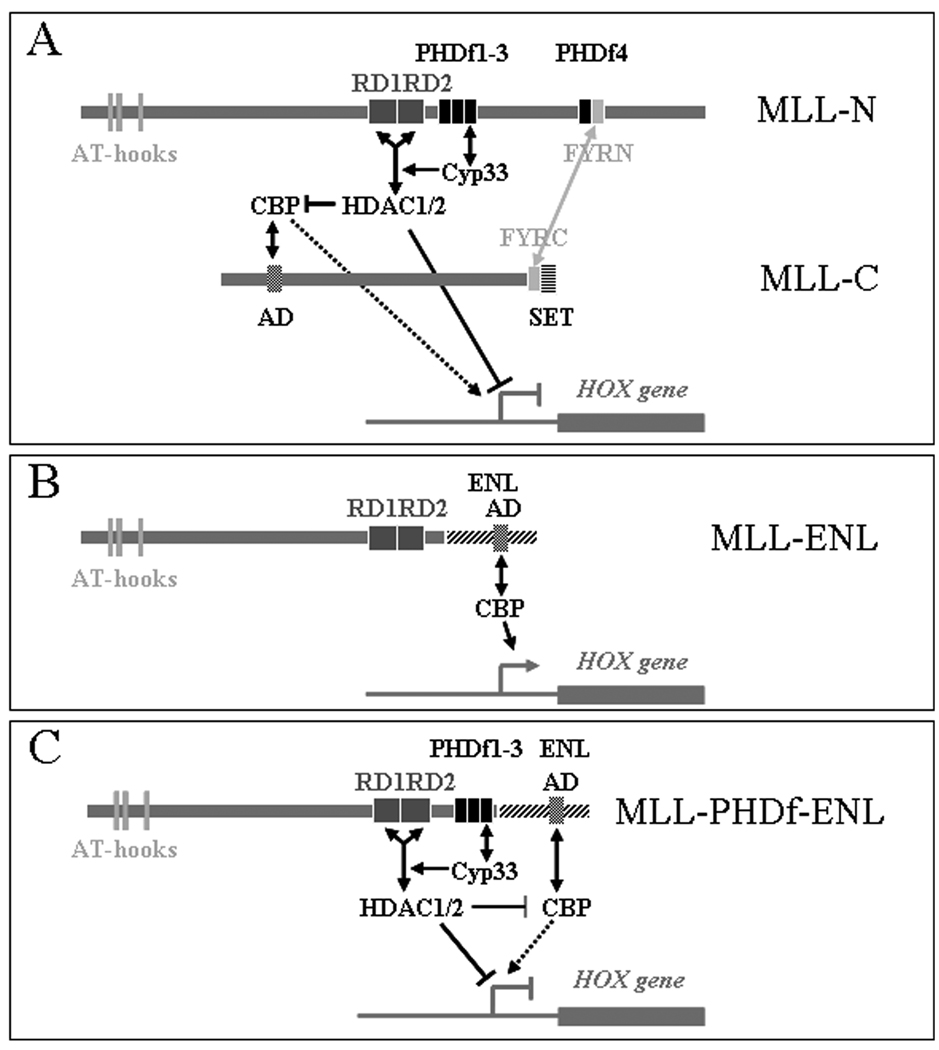
Figure 6. Diagram of a hypothetical model of HOX gene regulation by MLL and MLL-fusion proteins.
A: Wild type MLL is proteolytically cleaved and the two fragments associate within a larger protein complex through interaction of their FYRN and FYRC domains. Cyp33 binding to the 3rd PHD finger enhances the recruitment of HDAC1 and 2, resulting in inhibition of the MLL-associated CBP histone acetyltransferase activity and in histone deacetylation at the promoter of the target HOX genes. Depending on the binding of Cyp33, wild type MLL can switch between activation and repressing functions. B: The MLL-ENL fusion protein which has an activation domain on the ENL component that may also bind CBP, lacks the 3rd PHD finger and cannot bind Cyp33; thus, it cannot recruit HDAC1 and 2 and functions as a dominant constitutive activator. C: Reinsertion of the 3rd PHD finger restores the regulation of the fusion protein by Cyp33-HDACs restoring its function to regulated activation/repression.