Abstract
The ratio of divalent to monovalent ion concentration necessary to displace the surface-active protein, albumin, by lung surfactant monolayers and multilayers at an air-water interface scales as 2−6, the same concentration dependence as the critical flocculation concentration (CFC) for colloids with a high surface potential. Confirming this analogy between competitive adsorption and colloid stability, polymer-induced depletion attraction and electrostatic potentials are additive in their effects; the range of the depletion attraction, twice the polymer radius of gyration, must be greater than the Debye length to have an effect on adsorption.
INTRODUCTION
Monolayer films of lung surfactant (LS) line the alveolar air-water interface and lower the interfacial tension in the lungs, thereby minimizing the work of breathing 1, 2. However, in the complex physical and chemical environment of the lung, it is inevitable that surface-active species in the alveolar fluids or deposited from air compete with LS for the interface. One critical situation in which competitive adsorption may be important is acute respiratory distress syndrome (ARDS) 3–5. The roughly 140,000 patients in the US with ARDS have elevated levels of surface-active serum and inflammatory proteins in their bronchial fluid 4–22 that can compete with lung surfactant for the air-liquid interface of the alveolus 22–29. If the presence of the serum proteins at the interface inhibits or prevents the adsorption of LS, the surface tension control necessary for optimal respiration can be compromised, which may be partially responsible for the 40% mortality rates for ARDS.
Surface-active material adsorbs spontaneously to the air-water interface because adsorption lowers the interfacial energy. The surface tension, γ, is the derivative of the free energy, G, with respect to the interfacial area, A: 30. While adsorption of serum proteins such as albumin lowers the free energy, the lower equilibrium surface tension of LS (~ 25–30 mN/m 27) compared to albumin (~50 – 55 mN/m 31) suggests that, at equilibrium, LS should occupy the interface to the exclusion of albumin. However, LS adsorption, while favored energetically, does not occur for hours (if at all) when albumin or other surface-active serum proteins are in solution, or already occupy the interface 11, 27, 28.
This suggests that the kinetics of adsorption plays an important role. The initial rates of adsorption of LS and albumin from solution are determined by the relative sizes and diffusivities of LS and serum proteins. The surface active form of LS is composed primarily of disaturated dipalmitoylphosphatidylcholine (DPPC), with smaller fractions of unsaturated phospholipids and cholesterol, and two hydrophobic LS specific proteins 32, all of which have essentially no molecular solubility in physiological saline. As a result, LS self-assembles into bilayers in aqueous solution; these bilayers further organize into multilamellar liposome-like aggregates 1 – 100 microns in diameter 33. When the liposomes contact the air-water interface, the bilayers break down to form insoluble, Langmuir-type monolayers and multilayers (See Movie 1 in Supplementary Materials) 2. While the equilibrium surface pressure, Π, (Π = γw−γ ; γw is the surface tension of a clean air-water interface, 72 mN/m at 25° C, and γ the measured surface tension) of a LS film is 40 – 45 mN/m, as the available interfacial area is reduced in the alveolus during exhalation or on compression of the film in a Langmuir trough, the area per molecule of LS decreases and the surface pressure increases to ~ 70 mN/m before the film “collapses” 34–36 (Fig. 1a). These high surface pressures (low surface tensions) are essential to optimal lung function.
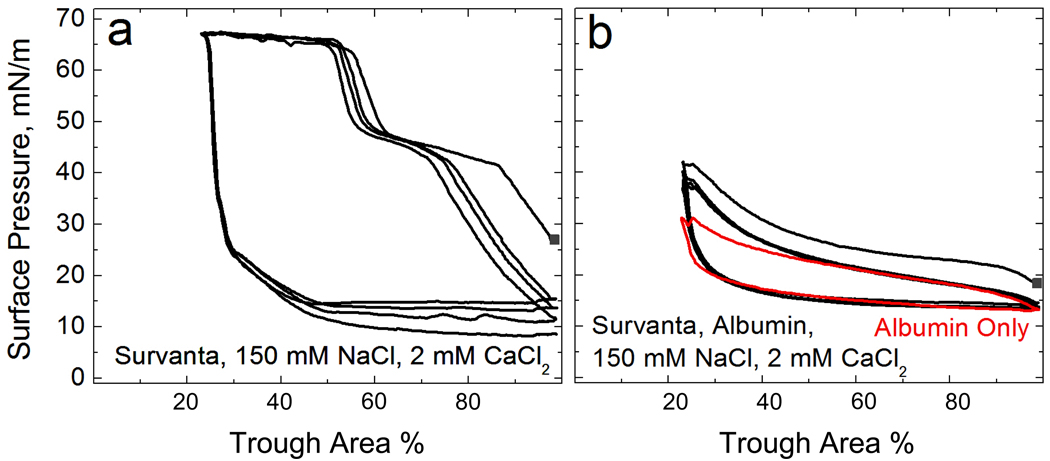
Figure 1.
(a) Surface pressure, (reduction in surface tension compared to a clean water interface with a surface tension of 72 mN/m) vs trough area cyclic isotherms of 800 µg lipid bilayer dispersion deposited on a buffered subphase containing no albumin (0.2 mM NaHCO3, 150 mM NaCl, 2 mM CaCl2, pH=7) in a Langmuir trough. On compression, the isotherm exhibits a characteristic shoulder at 45 mN/m and a collapse plateau at Πmax ~ 67 mN/m. On expansion, the surface pressure immediately drops to ~10–15 mN/m. The isotherms trace over each other on subsequent cycles.
(b) 800 µg lipid dispersion deposited onto an otherwise identical subphase containing 2 mg/mL albumin. The characteristic shoulder and collapse plateau on compression seen in (a) cannot be reached with albumin. The isotherms resemble that of albumin alone, shown in red, showing that lipid was prevented from adsorbing onto the interface 26.
Albumin, on the other hand, is a surface-active, but saline-soluble protein that forms a Gibbs-type monolayer with a surface pressure, Π, that is a logarithmic function of protein concentration up to a saturation concentration of ~1 mg/mL 31, 37. Albumin is a prolate spheroid of dimensions 4 × 4 × 14 nm; ellipsometry 38, neutron reflectivity 39 and x-ray reflectivity 40 indicate albumin forms a dense, closely packed monolayer 4–5 nm thick accompanied by a less dense second layer ~ 3 nm thick, with the albumin long axis parallel to the interface. The surface pressure at the saturation concentration for albumin and many other serum proteins is ~ 20 mN/m (γ ~ 50 mN/m) 31, 37, which is much lower than Π ~70 (γ near zero) required for proper respiration. Compression of an albumin film does not cause a significant increase in the surface pressure (Fig. 1b); the soluble albumin can leave the interface as the surface area decreases, thereby keeping the interfacial density and the surface pressure relatively constant. Because of its nanometer size and subsequent faster diffusion compared to the multi-micron bilayer liposomes of LS, albumin has significantly faster transport to (and from) the interface than the much larger LS liposomes 41. In both the expanding alveolus in the lung and the Langmuir trough, new air-water interface is continuously being created for this competitive adsorption. In addition to competing for this new interface, LS must displace albumin from whatever part of the interface already occupied for equilibrium to occur.
Even though energetically favorable, LS adsorption effectively ceases (Fig. 1b) when albumin is in solution or occupies the air-water interface 11, 27, 28, 31. Albumin (which is negatively charged at physiological pH) at the interface induces a steric and electrostatic energy barrier to diffusion of the LS liposomes, (which are also net negatively charged 29, 42), which prevents LS from reaching the interface and converting from bilayers to a surface-active film 28. This energy barrier to LS adsorption is reminiscent of the energy barrier that stabilizes colloidal dispersions and prevents them from flocculating 22, 25–29, 42, 43. Although it is well established that colloidal dispersions are only metastable, they can be prevented from flocculating (reaching equilibrium) for years if the energy barrier to aggregation is sufficiently high 41, 44–46.
Hence, it should not be surprising that the same additives used to lower the energy barrier to colloid aggregation 41, 47–53, also lead to enhanced surfactant adsorption in the presence of albumin or serum proteins. The first suggestion of the analogy between colloid stability and surfactant adsorption came from observations that adding non-ionic hydrophilic polymers such as polyethylene glycol (PEG) and dextran 54–58 or anionic polymers such as hyaluronic acid 59 to clinical lung surfactants improved lung function in animals with lung injuries. This improved lung function correlated with enhanced surfactant adsorption to an albumin-covered air-water interface in vitro, as well as the flocculation of the LS liposomes in suspension 24–28, 40, 43, 60. Both the enhanced adsorption 61 and flocculation 62 of the surfactant resulted from the depletion attraction that entropically pushes the surfactant aggregates toward the interface 61 and toward each other 62, thereby causing the excluded volumes of the aggregates and interface to overlap and increase the solution volume available to the polymer. This attractive interaction between interface and surfactant helps to lower the albumin-induced energy barrier 28.
A second method of enhancing LS adsorption, which is also commonly used in colloid flocculation, is to add the positively charged polyelectrolyte chitosan to the LS/albumin solution 15, 22, 29, 42. In analogy to its effects on colloid stability 48–53, chitosan enhances the adsorption of lung surfactant in vitro 15, 29 at concentrations orders of magnitude too low to exert an appreciable depletion attraction 42. However, increasing the chitosan concentration above optimal causes surfactant adsorption to decrease. This is similar to the initial rapid increase in colloid flocculation with polyelectrolyte concentration, followed by re-stabilization of colloidal dispersions at higher polyelectrolyte concentrations 48–53. The polycation initially neutralizes the anionic surfaces 48–53, which causes an elimination of the double-layer repulsion, which in turn, lowers the energy barrier to adsorption (or colloid aggregation). However, as is often the case for polyelectrolytes, higher polymer concentrations lead to over-compensation of the surface charge, which re-establishes the electrostatic energy barrier 48–53, leading to a decrease in surfactant adsorption, or re-stabilization of the colloid dispersion. This same physical mechanism is exploited in the “alternate layer deposition” of anionic and cationic polyelectrolytes on charged colloids 51.
In this work, we complete the analogy between colloid stability and competitive adsorption to interfaces by considering the effects of electrolyte concentration on adsorption. One of the first surprising generalizations regarding colloid stability (1880–1900) was that the critical electrolyte concentration required to flocculate (CFC) a variety of positive and negative colloids decreased as z−6, (z is the valence of the ion opposite in charge to the colloid), which is known as the Schulze-Hardy rule 41, 63, 64. Explaining this result first required the derivation by Smoluchowski (1917) of diffusion-limited aggregation 65, which Fuchs (1934) extended to show that the flocculation rate slowed in the presence of an repulsive potential, Φ 66. The ratio of the diffusion-limited flux, Jo, to the actual flux, J, is proportional to the exponential of the potential maximum, Φmax 67:
| (1) |
The ratio is known in the colloid literature as the stability ratio, W. The Derjaguin, Landau, 44 Verwey, Overbeek 45 (DLVO, 1940’s) theory combined the van der Waals/London dispersion attraction 68 with double-layer electrostatic repulsion 41 to give the functional form of Φ between two spheres of radius, a, at a separation, r, surface potential, ψs, and ion concentration, ni, via the Debye length, :
| (2) |
AH is the Hamaker constant that determines the magnitude of the attractive dispersion forces 68. Flocculation occurs at an ion concentration (called the critical flocculation concentration or CFC) at which Φmax = dΦ/dr = 0, which occurs when (r − 2a)= κ−1:
| (3) |
For ezψs/4kBT > 1, in the limit of large surface potentials, the CFC ∝z−6, which successfully explained the Schulze-Hardy rule 41, validating the DLVO theory. However, the z−6 dependence is actually rather uncommon; high electrolyte concentrations often lead to counterion adsorption to the colloid or interface, partially neutralizing ψs. For such lower surface potentials, ezψs/kBT ≪ 1, and the .
Eqns. 1–3 can also be used to predict the effects of electrolyte concentration and valence on the competitive adsorption of LS to the air-water interface in the presence of albumin. Increasing the electrolyte concentration increases surfactant adsorption to the air-water interface in the presence of albumin. In analogy to the CFC (Eqn. 3), the ratio of divalent (calcium) to monovalent (sodium) ion concentration needed to induce diffusion limited surfactant adsorption is proportional to 2−6, which is, as far as we are aware, the first demonstration of the Schulze-Hardy rule for competitive adsorption. On the other hand, lowering the electrolyte concentration sufficiently (to sub-physiological levels) can increase the magnitude and range of the repulsive interaction so as to bypass the range of the depletion attraction, which is limited to twice the polymer radius of gyration, which eliminates the effects of polymers on surfactant adsorption.
Materials and Methods
The clinical replacement LS, Survanta (Abbott Laboratories, Columbus, Ohio) was used as a model lung surfactant in all experiments and was a generous gift of the Santa Barbara Cottage Hospital nursery. Survanta is an organic extract of minced bovine lungs that has been fortified with dipalmitoylphosphatidylcholine (DPPC), tripalmitin and palmitic acid. Survanta contains 80 – 90% wt. phosphatidylcholine, of which, ~70% wt. is saturated DPPC and about 10% wt. palmitic acid 32, 69. The preparation contains approximately 7% wt. negatively charged phospholipids including phosphatidylglycerol and phosphatidylserine giving the Survanta aggregates a net negative charge 69. Survanta has minimal amounts of the LS specific amphipathic protein SP-B, 0.04–0.13% wt. but close to native amounts 0.9–1.65% wt., of the hydrophobic LS protein. SP-C, 32, 33, 70. Both SP-B and SP-C are cationic, which partially compensates the negative charge on the lipids. Like other natural products, Survanta can vary somewhat from batch to batch due to variations in extraction and purification as well as variations in the source materials. Survanta and other clinical lung surfactants form multi-micron bilayer aggregates in buffered saline solution 33. Bovine serum albumin and 10kDa polyethylene glycol (PEG) were obtained from Sigma (St. Louis, MO) and used as received.
Isotherms were recorded at 25°C (No significant changes are seen from 23 – 37°C 71) using a custom stainless steel ribbon trough (Nima, Coventry, England) designed to minimize film leakage at high surface pressures (low surface tensions). Surface pressure was monitored during compression and expansion using a filter paper Wilhelmy plate. The trough had a surface area of 130 cm2; a subphase volume of 150 mL and a typical compression/expansion cycle took 8 min (~0.42 cm2/sec). All water used in experiments was obtained from a Millipore Gradient System (Billerica, MA) and had a resistivity of 18.2 MΩ/cm. The buffered-saline subphase contained 150 mM NaCl and 0.2 mM NaHCO3 in addition to the stated concentrations of albumin and other electrolytes.
To initiate each experiment, a saline-buffered subphase was added to the Langmuir trough and allowed to equilibrate for 10 minutes. For albumin containing subphases, the surface pressure gradually increased to 15 –18 mN/m, consistent with the well-established relation between surface activity and albumin concentration 31, 37. PEG in buffer showed no surface activity 40. For all experiments, Survanta was diluted in a standard buffer (150 mM NaCl, 0.2 mM NaHCO3, pH=7.0) to a lipid concentration of 2 mg/mL and was deposited as microliter drops from a syringe by touching the drop to the air-water interface of the open trough. The drops passed through the interface into the subphase adjacent to the interface; surfactant spreading from the subphase was monitored by labelling the Survanta with 1 mol% of the fluorescent lipid Texas Red-DHPE (Invitrogen, Eugene, OR). The drops did not spread appreciably at the interface and essentially all of the Survanta adsorbed from the subphase 25. The subphase was not stirred and the first compression began 20 minutes after deposition of a fixed quantity of Survanta. The amount of Survanta chosen for the inhibition experiments, 800 µg, was such that collapse would occur at about 50% trough compression in the absence of albumin; the same amount of surfactant was used in all subsequent experiments.
A Nikon Optiphot optical microscope (Nikon, Tokyo, Japan) with either a 10X or 50X extra long working distance objective designed for fluorescent light 72 was positioned above the trough. Full-length movies and individual frames were recorded directly to computer (Moviestar, Mountain View, CA). Contrast in the images was due to segregation of 1% mol fluorescent lipid Texas Red-DHPE (Invitrogen, Eugene, OR) between the liquid expanded and condensed phases which causes the Survanta monolayer to have a light gray-dark gray coexistence in images 25, 71, 73. Larger aggregates of Survanta have significantly more dye than the monolayer film and appear bright white, leading to an overall mottled texture for the surfactant film. The albumin was not labeled, does not fluoresce and appears black in the images.
Results and Discussion
Fig. 1a shows typical surface pressure/area cyclic Langmuir isotherms for 800 µg of the Survanta dispersion adsorbed from a buffered subphase, (0.2 mM NaHCO3 and pH=7) containing 150 mM NaCl and 2 mM CaCl2, but no albumin 71. The isotherm traces over itself on subsequent cycles, and on compression (starting at ~ 80% trough area) exhibits a shoulder at Π ~ 45 mN/m 71. On further compression to 55–60% trough area, the surface pressure rises abruptly to the collapse pressure, Πmax ~ 67 mN/m, where the film begins to form cracks and folds 34, 36. This failure of the monolayer is what determines the minimum surface tension. On expansion of the films, the surface pressure drops rapidly; the hysteresis between compression and expansion cycles is typical of such isotherms. Grazing incidence X-ray diffraction shows that the area per molecule of Survanta is fixed for a given surface pressure and temperature, regardless of the subphase concentration 40. Hence, the fractional compression of the trough needed to reach these characteristic features is proportional to the amount of surfactant that adsorbs to the interface.
Under otherwise identical conditions, if 2 mg/mL albumin is in the subphase (Fig. 1b), the surface pressure does not increase above ~35 mN/m even at the smallest trough area. The characteristic shoulder and collapse plateau seen on compression in Fig. 1a cannot be reached with albumin in the subphase, signifying inhibited Survanta adsorption. The isotherm is essentially that of albumin alone (red curve) 25, 26. The albumin prevents any significant amount of Survanta from adsorbing to the interface over the approximately 4 hour length of this experiment 27. This is consistent with the anionic albumin creating an energy barrier to adsorption of the anionic Survanta bilayers, similar to the stabilization of a colloidal dispersion against flocculation by electrostatic repulsion.
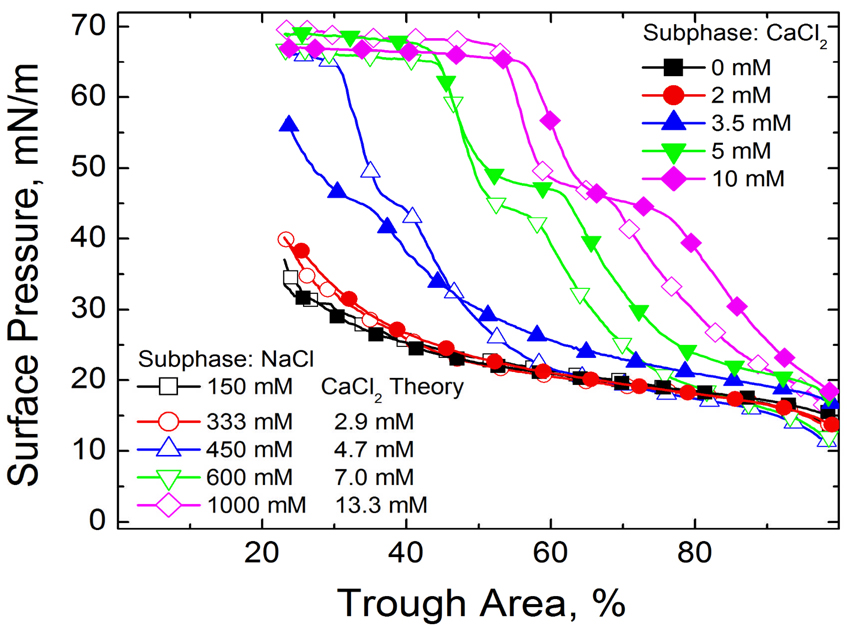
Fig. 2 shows that increasing the monovalent ion concentration converts the isotherm from an albumin-like isotherm at 150 mM (physiological) and 333 mM NaCl to that of Survanta on a albumin-free interface (compare to Fig. 1a) at 1000 mM NaCl. Just as in colloid stability, (See Eqn.2), increasing the electrolyte concentration decreases the Debye length and the range and magnitude of the electrostatic repulsion between the albumin and the surfactant. This lowers the energy barrier to surfactant adsorption; more surfactant reaches the interface to displace the albumin from the interface (See Movie in Supplemental Materials).
Figure 2.
The fourth cycle compression isotherms of 800 µg lipid dispersion on a buffered saline subphase (0.2 mM NaHCO3 and pH=7) containing 2 mg/mL albumin and varying electrolyte concentrations are plotted. □ 150 mM NaCl subphase; ○ 333 mM NaCl subphase; ∆ 450 mM NaCl subphase; ▽ 600 mM NaCl subphase; ◊ 1000 mM NaCl; ■ 0 mM CaCl2, 150 mM NaCl subphase; ● 2 mM CaCl2, 150 mM NaCl subphase; ▲ 3.5 mM CaCl2, 150 mM NaCl subphase; ▼ 5 mM CaCl2, 150 mM NaCl subphase; ♦10 mM CaCl2, 150 mM NaCl subphase. For each NaCl concentration, the theoretical CaCl2 concentration according to the Schulze-Hardy scaling is given.
Fig. 2 also shows that significantly lower levels of CaCl2 restore surfactant adsorption in the presence of albumin. For each NaCl concentration, the Schulze-Hardy rule (CFC proportional to z−6) is used to compare the isotherm with a functionally equivalent amount of CaCl2 in the subphase. To make the comparisons physiologically relevant, 150 mM NaCl was taken as a baseline electrolyte concentration (e.g. for 1000 mM total NaCl, the equivalent CaCl2 concentration for 850 mM NaCl is 2−6 * 850 = 13.3 mM). The CaCl2 concentrations relative to the NaCl concentrations (above 150 mM) to restore surfactant adsorption are in the ratio 2 −(6.4 ±0.1).
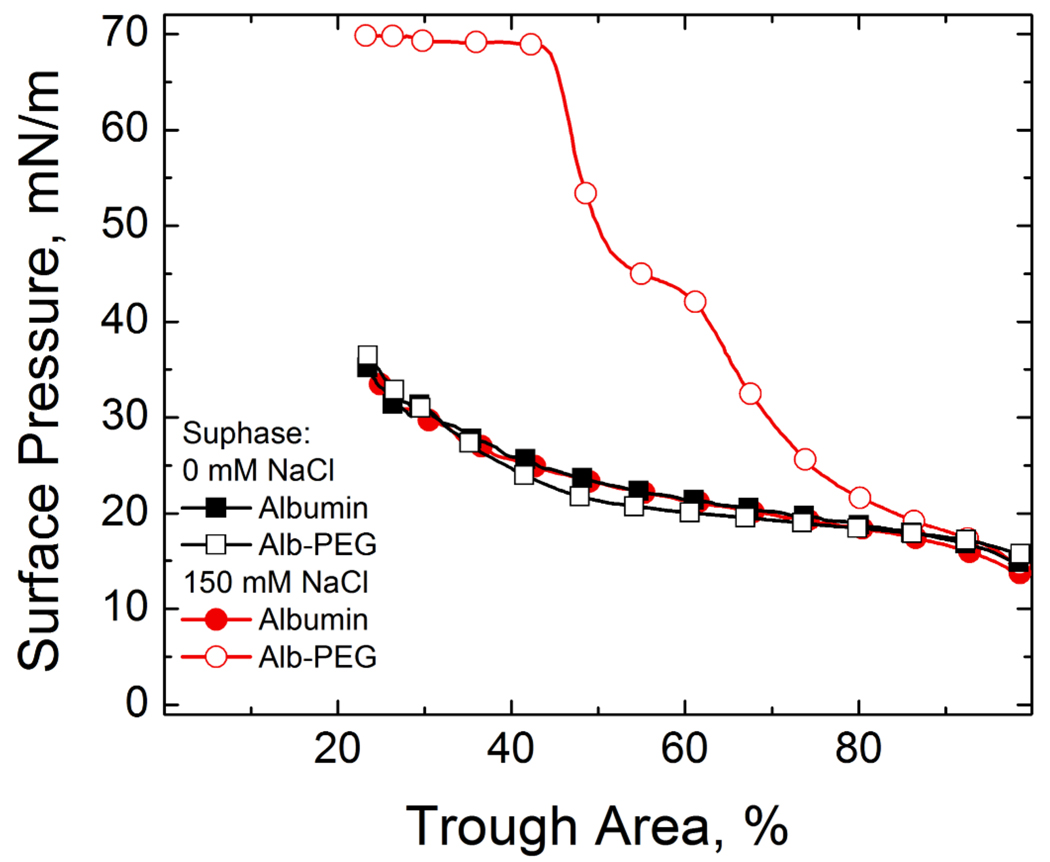
If the analogy between the CFC and competitive adsorption holds, other methods of changing Φ should also lead to enhanced adsorption. For example, adding 1 wt% 10 kDa PEG to a subphase containing 150 mM NaCl also restores surfactant adsorption in the presence of albumin (Fig. 3) 25, 26. This suggests that a second generic colloidal interaction, the so-called “depletion attraction”, assists in enhancing surfactant adsorption, just as it promotes colloid flocculation.
Figure 3.
Fourth cycle compression isotherms of 800 µg lipid dispersion on a buffered subphase (0.2 mM NaHCO3 and pH=7) containing 2 mg/mL albumin, 10 mg/mL 10 kDa PEG and varying NaCl concentrations. Filled symbols denote subphases containing albumin and NaCl while open symbols denote subphases containing albumin, NaCl and PEG. ■ 0 mM NaCl-albumin subphase; □ 0 mM NaCl- albumin-PEG subphase; ● 150 mM NaCl-albumin-subphase; ○ 150 mM NaCl-albumin-PEG subphase. Adding PEG restores the characteristic shoulder and collapse plateau of the Survanta with 150 mM NaCl in the subphase, but the same amount of PEG added to a 0 mM NaCl subphase does not alter the albumin-like isotherm. The range of the depletion attraction induced by PEG is twice the radius of gyration of the polymer, about 9 nm for 10 kDa PEG, which is less than the Debye length of 13 nm for the 0 mM NaCl subphase. For 150 mM NaCl, the Debye length is 1 nm, so the PEG induced depletion attraction has sufficient range to lower the repulsive potential.
The depletion attraction arises in mixtures of different sizes of non-interacting “hard spheres”. Here, the small spheres are the polymers with radius of gyration, Rg (~ 5 nm for 10 kDa PEG), and the large spheres are the surfactant aggregates of radius R (typically microns for Survanta, see movie in supplemental materials). As a large sphere of surfactant moves toward another large sphere of surfactant or the interface, the volume excluded from the centers of the small spheres (the PEG molecules) overlap, which causes the volume accessible to the small spheres to increase. This decreases the free energy of the mixture (or increases the entropy) by an amount proportional to the size of the excluded volume overlap region, multiplied by the osmotic pressure of the small spheres, which is proportional to the volume fraction of polymer, ϕp. This acts like an attractive potential between the large sphere and the interface, known as the depletion attraction, Φdep:
| (4) |
for r < 2Rg and Φdep = 0 for r > 2Rg. When Eqn. 4 is added to the DLVO potential in Eqn.2, the effective Φmax apparently goes to zero at 150mM salt concentration and the surfactant adsorbs as to a clean interface (Fig. 3). From Fig. 2, without the polymer, 1000 mM salt is required to restore surfactant adsorption; hence, 1 wt% 10 kDa PEG provides as much decrease in Φmax as ~ 800 mM salt.
However, for a subphase with no added salt, 1 wt% PEG did not lead to any increase in the adsorption of surfactant in the presence of albumin. This is because the maximum in the DLVO potential (Eqn. 2) occurs when the separation between the charged surfaces, (r−2a) is of order κ−1. For the subphase with no added NaCl, the 0.2 mM NaHCO3 buffer gives a κ−1 of 12 – 13 nm. The maximum range of the depletion attraction, 2Rg, however, is only ~ 10 nm for 10 kDa PEG 25, 26, 28. Hence the range of the depletion attraction is not sufficient, regardless of the polymer concentration, to affect Φmax, and PEG has no effect on adsorption (Fig. 3), as observed. For 150 mM salt, κ−1 ~ 1 nm, 2Rg ≫ κ−1 and the range and magnitude of the depletion attraction is sufficient to lower Φmax enough to restore diffusion-limited adsorption 25, 26.
This analogy between competitive adsorption and colloid stability has not been demonstrated quantitatively before. From Fig. 2, the Schulze-Hardy rule holds for competitive adsorption: the ratio of calcium to sodium concentration necessary to restore surfactant adsorption scales as 2−6. Polymer-induced depletion attraction and the double layer electrostatic potential are additive in their effects on adsorption; however, the range of the depletion attraction, which is limited to 2Rg, must be sufficient to overlap with the maximum in the electrostatic repulsion that occurs at ~ κ−1 to have an effect. These results may have important physiological consequences. In Acute Respiratory Distress Syndrome (ARDS), increased levels of serum proteins, such as albumin, in the lung due to injury or disease compete with lung surfactant for the available air-water interface 25, 27, 28, 31, 43. Insufficient surfactant at the interface does not allow the low surface tensions required for proper lung function to be reached during the normal cycle of breathing. Future ARDS treatment may take advantage of low concentrations of divalent or multivalent ions or hydrophilic proteins to enhance lung surfactant adsorption in the presence of serum proteins.
Supplementary Material
Movie: Fluorescence optical microscopy of Survanta labeled with 1% mol Texas Red-DHPE (Invitrogen, Eugene, OR) (gray to bright white) adsorbing to an interface covered by albumin (black). The Survanta monolayer film is a mottled gray with white patches that spreads and displaces the albumin at the interface when 1000 mM NaCl is in the subphase (See Fig. 2). The fluffy white patches are aggregates of Survanta liposomes loosely attached to the interface. When the Survanta liposomes contact the interface, they fuse and spread to form the monolayer film. This information is available free of charge via the Internet at http://pubs.acs.org/.
Acknowledgements
Support for this work comes from National Institute of Health Grant HL-51177 and the Tobacco Related Disease Research Program 14RT-0077. P.C.S. was partially supported by an NSF graduate research fellowship.
References
- 1.Clements JA, Avery ME. Amer. J. Respir. Crit. Care Med. 1998;157:S59–S66. doi: 10.1164/ajrccm.157.4.nhlb1-1. [DOI] [PubMed] [Google Scholar]
- 2.Notter R. Lung Surfactant: Basic Science and Clinical Applications. Vol. 149. New York: Marcel Dekker; 2000. [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. New England Journal of Medicine. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Gil J. Biochim. Biophys. Acta. 2008;1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt R, Markart P, Ruppert C, Wygrecka M, Kuchenbuch T, Walmrath D, Seeger W, Guenther A. Respiratory Res. 2007;8:55. doi: 10.1186/1465-9921-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Da Silva K, McCaig LA, Veldhuizen RA, Possmayer F. Exp. Lung Res. 2005;31:745–758. doi: 10.1080/01902140500267431. [DOI] [PubMed] [Google Scholar]
- 7.Dahlem P, van Aalderen WMC, Bos AP. Ped. Respir. Rev. 2007;8:348–362. doi: 10.1016/j.prrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Davidson WJ, Dorscheid D, Spragg R, Schulzer M, Mak E, Ayas NT. Crit. Care. 2006;10:R41–R45. doi: 10.1186/cc4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunasekara L, Schoel WM, Schürch S, Amrein MW. Biochim. Biophys. Acta. 2008;1778:433–444. doi: 10.1016/j.bbamem.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Haitsma JJ, Papadakos PJ, Lachmann B. Curr. Opinion Critical Care. 2004;10:18–22. doi: 10.1097/00075198-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Holm BA, Enhorning G, Notter RH. Chem. Phys. Lipids. 1988;49:49–55. doi: 10.1016/0009-3084(88)90063-1. [DOI] [PubMed] [Google Scholar]
- 12.Holm BA, Notter RH, Finkelstein JN. Chem. Phys. Lipids. 1985;38:287–298. doi: 10.1016/0009-3084(85)90022-2. [DOI] [PubMed] [Google Scholar]
- 13.Holm BA, Wang WD, Notter RH. Ped. Res. 1999;46:85–93. doi: 10.1203/00006450-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaka A, Matsuda T, Albertine KH, Koh H, Tasaka S, Hasegawa N, Kohno N, Kotani T, Morisaki H, Takeda J, Nakamura M, Fang XH, Martin TR, Matthay MA, Hashimoto S. Amer. J. Physiol. 2004;286:L1088–L1094. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 15.Kang NX, Policova Z, Bankian G, Hair ML, Zuo YY, Neumann AW, Acosta E. J.,Biochim. Biophys. Acta. 2008;1778:291–302. doi: 10.1016/j.bbamem.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Kesecioglu J, Haitsma JJ. Current Opin. Critical Care. 2006;12:55–60. doi: 10.1097/01.ccx.0000199000.19393.a7. [DOI] [PubMed] [Google Scholar]
- 17.Larsson M, Nylander T, Keough KMW, Nag K. Chem. Phys. Lipids. 2006;144:137–145. doi: 10.1016/j.chemphyslip.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Notter RH, Schwan AL, Wang Z, Waring AJ. Mini Rev. Med. Chem. 2007;7:932–944. doi: 10.2174/138955707781662627. [DOI] [PubMed] [Google Scholar]
- 19.Raghavendran K, Pryhuber GS, Chess PR, Davidson BA, Knight PR, Notter RH. Curr. Med. Chem. 2008;15:1911–1924. doi: 10.2174/092986708785132942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willson DF, Chess PR, Notter RH. Pediatr. Clin. North Am. 2008;55:545–575. doi: 10.1016/j.pcl.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, Jacobs BR, Jefferson LS, Conaway MR, Egan EA. JAMA. 2005;293:470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 22.Zuo YY, Veldhuizen RA, Neumann AW, Peterson NO, Possmayer F. Biochim. Biophys. Acta. 2008;1778:1947–1977. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Stenger PC, Palazolgu O, Zasadzinski JA. In: The mechanism of chitosan lung surfactant adsorption at the air-liquid interface in the presence of serum proteins. Tok JB, editor. Vol. 1061E. Boston: MRS Society; 2008. [Google Scholar]
- 24.Stenger PC, Wu G, Chi EY, Frey SL, Lee KYC, Majewski J, Kjaer K, Zasadzinski JA. In: Competitive adsorption of lung surfactant and serum proteins at the air-liquid inerface: a grazing incidence X-ray diffraction study. Thompson C, Durr H, Toney M, Noh D, editors. Vol. 1027E. Boston: MRS Society; 2008. [Google Scholar]
- 25.Stenger PC, Zasadzinski JA. Biophys. J. 2007;92:3–9. doi: 10.1529/biophysj.106.091157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenger PS, Isbell SG, Zasadzinski JA. Biochim. Biophys. Acta. 2008;1778:2032–2040. doi: 10.1016/j.bbamem.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taeusch HW, de la Serna JB, Perez-Gil J, Alonso C, Zasadzinski JA. Biophys. J. 2005;89:1769–1779. doi: 10.1529/biophysj.105.062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zasadzinski JA, Alig TF, Alonso C, de la Serna JB, Perez-Gil J, Taeusch HW. Biophys. J. 2005;89:1621–1629. doi: 10.1529/biophysj.105.062646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo YY, Alolabi H, Shafiei A, Kang NX, Policova Z, Cox PN, Acosta E, Hair ML, Neumann AW. Ped. Res. 2006;60:125–130. doi: 10.1203/01.pdr.0000227558.14024.57. [DOI] [PubMed] [Google Scholar]
- 30.Marsh D. Biochim. Biophys. Acta. 1996;1286:183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 31.Warriner HE, Ding J, Waring AJ, Zasadzinski JA. Biophys. J. 2002;82(2):835–842. doi: 10.1016/S0006-3495(02)75445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernhard W, Mottaghian J, Gebert A, Rau GA, von der Hardt H, Poets CF. Amer. J. Respir. Crit. Care Med. 2000;162:1524–1533. doi: 10.1164/ajrccm.162.4.9908104. [DOI] [PubMed] [Google Scholar]
- 33.Braun A, Stenger PC, Warriner HE, Zasadzinski JA, Lu KW, Taeusch HW. Biophys. J. 2007;93:123–139. doi: 10.1529/biophysj.106.095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipp MM, Lee KYC, Takamoto DY, Zasadzinski JA, Waring AJ. Phys. Rev. Lett. 1998;81:1650–1653. [Google Scholar]
- 35.Lu W, Knobler CM, Bruinsma RF, Twardos M, Dennin M. Phys. Rev. Lett. 2002;89:146107. doi: 10.1103/PhysRevLett.89.146107. [DOI] [PubMed] [Google Scholar]
- 36.Pocivavsek L, Dellsy R, Kern A, Johnson S, Lin B, Lee KYC, Cerda E. Science. 2008;320:912–916. doi: 10.1126/science.1154069. [DOI] [PubMed] [Google Scholar]
- 37.Krishnan A, Sturgeon J, Siedlecki CA, Vogler EA. J. Biomed. Materials Res. A. 2004;68A:544–557. doi: 10.1002/jbm.a.20104. [DOI] [PubMed] [Google Scholar]
- 38.McClellan SJ, Franses EI. Coll. Surf. B. 2003;28:63–75. [Google Scholar]
- 39.Lu JR, Su TJ, Penfold J. Langmuir. 1999;15:6975–6983. [Google Scholar]
- 40.Stenger PC, Wu G, Miller CE, Chi EY, Frey SL, Lee KYC, Majewski J, Kjaer K, Zasadzinski JA. Biophys. J. 2009 doi: 10.1016/j.bpj.2009.05.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russel WB, Saville DA, Schowalter WR. Colloidal Dispersions. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- 42.Stenger PC, Palazolgu O, Zasadzinski JA. Biochim. Biophys. Acta. 2009;1788:1033–1043. doi: 10.1016/j.bbamem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu LMY, Lu JJ, Chiu IWY, Leung KS, Chan YWW, Zhang L, Policova Z, Hair ML, Neumann AW. Coll. Surf. B. 2004;36:167–176. doi: 10.1016/j.colsurfb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Derjaguin BV, Landau L. Acta Physicochim. URSS. 1941;14:633–662. [Google Scholar]
- 45.Verwey EJW, Overbeek JTG. Theory of the Stability of Lyophobic Colloids. Amsterdam: Elsevier; 1948. [Google Scholar]
- 46.Israelachvili JN. Intermolecular and Surface Forces. 2nd Ed. ed. ed. London: Academic Press; 1992. [Google Scholar]
- 47.Hiemenz PC. Principles of Colloid and Surface Chemistry. 2nd ed. New York: Marcel Dekker, Inc.; 1986. [Google Scholar]
- 48.Ashmore M, Hearn J. Langmuir. 2000;16:4906–4911. [Google Scholar]
- 49.Ashmore M, Hearn J, Karpowicz F. Langmuir. 2001;17:1069–1073. [Google Scholar]
- 50.Bouyer F, Robben A, Yu WL, Borkovec M. Langmuir. 2001;17:5225–5231. [Google Scholar]
- 51.Decher G. Science. 1997;277:1232–1237. [Google Scholar]
- 52.Kleimann J, Gehin-Delval C, Auweter H, Borkovec M. Langmuir. 2005;21:3688–3698. doi: 10.1021/la046911u. [DOI] [PubMed] [Google Scholar]
- 53.Lowack K, Helm CA. Macromolecules. 1998;31:823–833. [Google Scholar]
- 54.Kobayashi T, Ohta K, Tashiro K, Nishizuka K, Chen WM, Ohmura S, Yamamoto K. J. Appl. Physiol. 1999;86:1778–1784. doi: 10.1152/jappl.1999.86.6.1778. [DOI] [PubMed] [Google Scholar]
- 55.Lu JJ, Cheung WWY, Yu LMY, Policova Z, Li D, Hair ML, Neumann AW. Respiratory Physiology & Neurobiology. 2002;130:169–179. doi: 10.1016/s0034-5687(02)00006-3. [DOI] [PubMed] [Google Scholar]
- 56.Lu KW, Taeusch HW, Robertson B, Goerke J, Clements JA. Amer. J. Respir. Crit. Care Med. 2000;162:623–628. doi: 10.1164/ajrccm.162.2.9909099. [DOI] [PubMed] [Google Scholar]
- 57.Lu KW, Taeusch HW, Robertson B, Goerke J, Clements JA. Amer. J. Respir. Crit. Care Med. 2001;164:1531–1536. doi: 10.1164/ajrccm.164.8.2104016. [DOI] [PubMed] [Google Scholar]
- 58.Taeusch HW, Lu KW, Goerke J, Clements JA. Amer. J. Respir. Crit. Care Med. 1999;159:1391–1395. doi: 10.1164/ajrccm.159.5.9808047. [DOI] [PubMed] [Google Scholar]
- 59.Lu KW, Goerke J, Clements JA, Taeusch HW. Ped. Res. 2005;58:206–210. doi: 10.1203/01.PDR.0000169981.06266.3E. [DOI] [PubMed] [Google Scholar]
- 60.Lu KW, Goerke J, Clements JA, Taeusch HW. Ped. Res. 2005;57:237–241. doi: 10.1203/01.PDR.0000150726.75308.22. [DOI] [PubMed] [Google Scholar]
- 61.Kaplan PD, Rouke JL, Yodh AG, Pine DJ. Phys. Rev. Lett. 1994;72:582–585. doi: 10.1103/PhysRevLett.72.582. [DOI] [PubMed] [Google Scholar]
- 62.Asakura S, Oosawa F. J. Polymer Science. 1958;33:183–192. [Google Scholar]
- 63.Hardy WB. Proc. Roy. Soc. Lon. 1900;66:110–125. [Google Scholar]
- 64.Schulze H. J. Prakt. Chem. 1882;25:431–452. [Google Scholar]
- 65.Smoluchowski Mv. Z. Phys. Chem. 1917;92:129–168. [Google Scholar]
- 66.Fuchs N. Z. Phys. 1934;89:736–743. [Google Scholar]
- 67.Reerinck H, Overbeek JTG. Disc. Far. Soc. 1954;18:74–84. [Google Scholar]
- 68.Mahanty J, Ninham BW. Dispersion Forces. New York: Academic Press; 1976. [Google Scholar]
- 69.Blanco O, Perez-Gil J. Eur. J. Pharmacol. 2007;568:1–15. doi: 10.1016/j.ejphar.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 70.Walther FJ, Gordon LM, Zasadzinski JA, Sherman MA, Waring A. Neonatology. 2007;91:303–310. doi: 10.1159/000101346. [DOI] [PubMed] [Google Scholar]
- 71.Alonso C, Alig T, Yoon J, Bringezu F, Warriner H, Zasadzinski JA. Biophys. J. 2004;87:4188–4202. doi: 10.1529/biophysj.104.051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lipp MM, Lee KYC, Waring A, Zasadzinski JA. Biophys. J. 1997;72:2783–2804. doi: 10.1016/S0006-3495(97)78921-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McConnell HM. Ann. Rev. of Phys. Chem. 1991;42:171–195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie: Fluorescence optical microscopy of Survanta labeled with 1% mol Texas Red-DHPE (Invitrogen, Eugene, OR) (gray to bright white) adsorbing to an interface covered by albumin (black). The Survanta monolayer film is a mottled gray with white patches that spreads and displaces the albumin at the interface when 1000 mM NaCl is in the subphase (See Fig. 2). The fluffy white patches are aggregates of Survanta liposomes loosely attached to the interface. When the Survanta liposomes contact the interface, they fuse and spread to form the monolayer film. This information is available free of charge via the Internet at http://pubs.acs.org/.