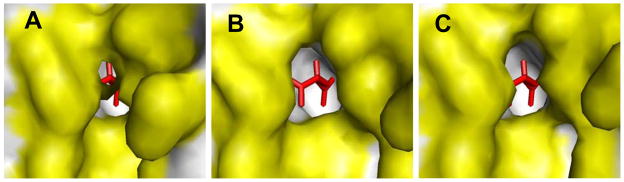
Figure 5.
(A) Close-up view of the active site of RacE2 as seen in the crystal structure. Residues lining the active site are colored yellow. D-Glu (red) is present inside the active site and cannot exit the active site without the active site opening. (B) Open active site of RacE2 as seen in one of the frames in mode 7 of the monomer (NMA results). (C) Open active site of RacE2 as seen in one of the frames in mode 10 of the dimer (NMA results). In the dimer, movement between the two domains of the monomer were observed in modes 10 and above, however the accessibility of the active site is less than that seen in the case of the monomer. This figure was generated by PyMOL.