Abstract
BACKGROUND
The effects of soy isoflavones on prostate cancer may be concentration dependent. The impact of soy supplementation on isoflavone concentrations in prostate tissues and serum remain unclear.
OBJECTIVE
To assess and compare concentrations of soy isoflavones in prostate tissue and serum among nineteen men with prostate cancer who had elected to undergo radical prostatectomy.
METHODS
Participants were randomized to receive either daily soy supplements (82 mg/day aglycone equivalents) or placebos for two weeks (14 days) prior to surgery. Serum samples were obtained at the time of the surgery. Isoflavone concentrations were measured by HPLC/ESI-MS-MS.
RESULTS
The median (25th, 75th percentile) total isoflavone concentration in the isoflavone supplemented group was 2.3 μmol/L (1.2, 6.9) in the prostate tissue and 0.7 μmol/L (0.2, 1.2) in the serum. Total isoflavone concentrations in this group were an average of ∼6-fold higher in prostate tissue compared to serum; the tissue vs. serum ratio was significantly lower for genistein than daidzein, 4-fold vs. 10-fold, p=0.003. Tissue and serum levels of isoflavones among the placebo group were negligible with a few exceptions.
CONCLUSIONS
The findings from the present study suggest that prostate tissue may have the ability to concentrate dietary soy isoflavones to potentially anti-carcinogenic levels. (199 words, 8/19/08)
INTRODUCTION
Prostate cancer (PCA) is the most common non-cutaneous cancer and is the second leading cause of cancer-related deaths among American men (1). The high incidence of preceding preneoplastic lesions and putative modifiable risk factors such as diet suggest opportunities for prostate cancer prevention. In fact, PCA is an ideal candidate for not only primary but also secondary prevention due to the long latency until the disease becomes clinically apparent. One dietary factor that has exhibited substantial potential for benefit is the isoflavone component of soy foods.
A growing body of evidence has established a strong association between diet and the incidence of PCA (2,3). Soy foods in particular have been linked to an inverse association with prostate cancer development (4). Asians consuming a traditional diet rich in soy products have lower morbidity and mortality from PCA than Europeans and U.S citizens (5). Asian immigrants to the U.S adopting a Western diet assume risks comparable to non-Asian Americans (6). These data suggest that dietary and/or environmental factors influence prostate cancer development or progression. Isoflavones in soy foods have been implicated as a critical micronutrient in cancer prevention and show broad anticancer activities (7-17).
Isoflavones, as a subclass of isoflavonoids, are plant-derived compounds exerting estrogen-like effects and are therefore classified as phytoestrogens. Activation of androgen signalling pathways is central to prostate carcinogenesis and anti-androgenic and estrogenic compounds have been exploited therapeutically in prostate cancer management for decades. In light of evidence of the anti-cancer and anti-androgen activity of isoflavonoids (18), nutritional supplementation may specifically have benefits in patients with PCA.
Only a limited number of clinical intervention trials have been conducted to determine the efficacy of isoflavone supplementation in PCA patients. One study reported a decrease in PSA (prostate-specific antigen) levels and lowered incidence of progression to PCA in patients with high-grade prostatic intraepithelial neoplasia (PIN) in response to soy supplements administered in a primary prevention setting (19). Others have reported decreased PCA detection after soy supplementation among high risk patients (20). However, several trials have shown negligible benefits in soy supplemented subjects (21-23). Some of the discrepancies in these findings are likely attributable to differences in study design, sample sizes, doses administered, and/or isoflavone concentrations achieved in the body (24).
It has been postulated that in vivo concentrations of isoflavone achievable from dietary sources may be lower than the concentration required for achieving anti-cancer effects (25,26). High isoflavone concentrations are easier to achieve in cell culture and in animal models than in humans through dietary administration. In cell culture studies, concentrations of 10 μM or higher have been demonstrated to decrease cell growth (27-29). In contrast, concentrations in cell culture studies of ≤1 μM have been observed to be proliferative (28), although Hedlund et al. and Swami et al. reported no difference in cell growth in the low concentration range (27,29). To date, no clinical studies have reported procarcinogenic effects specific to low concentrations of isoflavones.
Isoflavone concentrations in humans are typically determined from blood samples and levels of approximately 1 μM are considered to be the highest achievable through oral administration of natural food sources (30). Blood concentrations, however, are likely of lesser relevance than tissue levels. Farhan et al. reported a 10-fold higher concentration of isoflavone in prostate tissue than in serum taken from 4 mice given 250 μg of genistein by gastric tube (31). A few studies have directly assessed the isoflavone concentrations in the human prostatic fluid, or tissue (32-35). Among the 3 studies that compared serum with tissue concentrations, only two studies were designed as nutritional intervention trials and resulted in contradictory results (33,35). The latest study by Guy et al. observed tissue isoflavone concentrations to be lower than serum. Conversely, Rannikko et al. reported in a previous study a two-fold higher tissue isoflavone concentration compared to the corresponding serum concentration. The purpose of this double-blind, randomized controlled trial was to investigate the concentrations of soy isoflavones in prostatic tissues and serum among patients diagnosed with PCA undergoing curative surgical therapy who were given soy isoflavones or placebos for at least two weeks prior to prostatectomy.
MATERIALS AND METHODS
Patient selection
Patients with PCA who had elected to undergo radical prostatectomy were recruited between June 2004 and August 2005 through the urology clinic of investigator JDB at the Stanford University Hospital. Eligibility criteria included: newly diagnosed adenocarcinoma by prostate needle biopsy, clinically localized disease, and no hormone therapy or radiation prior to surgery. All participants provided written informed consent and the study was approved by the Stanford University Human Subjects Committee.
Experimental design
The study was a double-blind, randomized, placebo controlled trial. The participants were randomized to receive either soy supplements or a placebo. These supplements were consumed for two weeks (14 days) prior to the radical prostatectomy with two exceptions: one participant in the placebo group received pills for 13 days and one in the treatment group received supplements for 12 days. Serum was obtained on the day of the surgery.
Isoflavone and placebo supplements
The isoflavone supplement (NovaSoy) and matching placebo were generously provided by the Archer Daniels Midland company. The isoflavone content of the NovaSoy tablets, as determined in the laboratory of co-investigator AF, was 27.2 mg/tablet of total isoflavone (aglycone equivalent). The breakdown of individual isoflavones/tablet (aglycone equivalent) was 10.6 mg genistein, 13.3 mg daidzein, 3.2 mg glycitein, with negligible contributions from other forms.occurring as glucosides (83%), acetylglucosides (11%), and aglycones (6%). The placebo tablets were determined to have isoflavone content below the detection limit (<0.02015 mg total isoflavone aglycone equivalent/tablet). The placebo tablets were designed to look identical to the isoflavone tablets, and were composed of the same excipient compounds as that of the NovaSoy tablets. Adherence to the study regimen was determined by tablet count from returned bottles.
Prostate tissue extraction
The genistein, daidzein and equol content in prostatic tissue was determined from 50-100 mg of frozen tissue that was transferred into a 13×100mm test tube. After the addition of 0.5 mL water, the tissue was homogenized with a Brinkman homogenizer (Polytron, Switzerland) followed by the addition of 0.01 mL (400 ng/mL) of 13C triply labeled daidzein, genistein, and equol as internal standards (purchased from the University of St. Andrews, UK). This mixture was incubated with 0.1 mL of a mixture of protease (2.93 U/mL, Sigma, St. Louis, MO) and collagenase (242 U/mL, Sigma, St. Louis, MO) overnight. This enzyme treatment was shown not to hydrolyze isoflavonoid conjugates. After extraction with 3 mL of diethyl ether, the organic phase was removed, dried under nitrogen, re-dissolved with 0.8 mL methanol and 0.2 mL sodium acetate buffer (0.2 M; pH5), and centrifuged; 0.025 mL of clear supernatant was injected into the LC/MS system for isoflavonoid aglycone quantification. The aqueous phase was incubated with 0.02 mL glucuronidase (Escherichia coli 140 U/mL; Roche Applied Science, Indianapolis, IN) and 0.02 mL arylsulfatase (Helix pomatia, 5U/mL; Roche Applied Science, Indianapolis, IN,) for 3 hours at 37°C. After the addition of 0.01 mL (400 ng/mL) of 13C triply labeled daidzein, genistein, and equol as internal standards and 0.5 mL of 6N HCl, this mixture was exposed in a heat block to 100° C for 15 minutes. After cooling, isoflavonoids were extracted and injected into the LC/MS system for conjugate quantification as described below. The results of prostatic tissue analyses were expressed in nanomoles per kilogram wet weight.
High-Pressure Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry Analysis (HPLC/ESI-MS-MS)
LC/ESI-MS-MS was performed using a Finnigan TSQ Quantum triple quadruple mass spectrometer coupled to a Surveyor HPLC system (Thermo Electron Corporation, San Jose, CA). The HPLC and mass spectrometer were controlled by Xcalibur software (Thermo Electron Corporation). Liquid chromatography was carried out on a Beta Basic-8 column (100 mm × 2.1 mm i.d., 3 μm; Thermo Electron Corporation). A total of 25 μL of each sample was injected onto the column. The mobile phase, operating at a flow rate of 200 μL/min, consisted of methanol as solvent A, water as solvent B, and acetonitrile as solvent C. For the analysis of daidzein, genistein and equol, a linear gradient was used from 10/80/10 to 33/34/33 over 6 min. This was held for 1 min. The column was re-equilibrated with a mobile phase 10/80/10 for 3 min prior to the next injection.
High resolution selected reaction monitoring (HRSRM) of the deprotonated molecules [M-H] of daidzein, genistein and equol, and their diagnostically valuable product masses plus their isotopically labeled internal standards were measured for quantitation purposes.
Statistical analysis
Sample size estimates were determined on the basis of parameters available from previous studies. (36) Assuming a standard deviation of serum isoflavone concentrations of ∼1.4 μmol/L and a fasting serum isoflavone concentration of ∼0.5 μmol/L, a sample size of n=8/group was determined to be sufficient to detect a 5-fold difference between serum and prostate tissue isoflavone concentrations. Statistical analysis was performed using GraphPad Prism 5 software (GraphPad, San Diego, CA) and SPSS (version 16.0; SPSS Inc., Chicago, IL, USA). Differences between the placebo and soy-supplemented group were assessed with Mann-Whitney U-test for nonparametric variables and Student t tests for parametric variables as determined by the normality of the variable distribution. Descriptive statistics for non-normally distributed variables are presented using median values with 25th and 75th percentiles. Differences between paired serum and tissue samples for each individual were analyzed using Wilcoxon rank-sum test. Correlations were quantified by Spearman’s rank coefficient. Ratios between tissue and serum isoflavones are normally distributed and expressed as mean ± SD. All tests were two-tailed with a CI of 95%. P values <0.05 were considered statistically significant.
RESULTS
Patient characteristics, compliance and adverse events
A total of 25 patients were enrolled in the study; 14 patients were randomized to isoflavone group, and 11 patients received a placebo. Only data from subjects with both serum and prostate tissue samples were included in the analysis; serum samples were unavailable for 5 participants and tissue samples were unavailable for one participant, leaving 19 subjects available for analysis. Table 1 shows the demographic and clinical characteristics of the study cohort. No treatment related adverse effects were observed or reported during the study. The range of adherence to tablet consumption was 86-100% among the treatment and the placebo group. The mean ± SEM adherence was identical for both treatment and placebo group, 97% ± 2%.
Table 1.
Patient characteristics
| Placebo | Isoflavone | ||
|---|---|---|---|
| Total | 8 | 11 | |
| Age | Mean ± SD | 60.8 ± 7.7 | 55.9 ± 9.5 |
| PSA | Mean ± SD | 7.4 ± 3.9 | 5.2 ± 2.3 |
| cT | |||
| T1c | 4 | 7 | |
| T2a | 3 | 4 | |
| T2b | 1 | ||
| pT | |||
| T2a | 1 | 1 | |
| T2b | 3 | 9 | |
| T2c | 1 | ||
| T3a | 2 | ||
| T3b | 2 | ||
| Gleason | |||
| 3+3 | 2 | 1 | |
| 3+4 | 1 | 10 | |
| 4+3 | 1 | ||
| 4+5 | 4 | ||
| R | |||
| R0 | 6 | 10 | |
| R1 | 2 | 1 | |
PSA = Prostate-specific antigen
TNM classification: cT = clinical tumor category, pT = surgical-pathological tumor category, R = residual tumor
Tissue concentrations of isoflavones
The tissue concentration (median; 25th, 75th percentiles) of isoflavone conjugates were 0.09 μmol/kg (0.08, 0.28) in the placebo compared to 2.31 μmol/kg (1.16, 6.86) in the isoflavone group (p<0.001). For genistein and daidzein the tissue concentrations were 0.009 μmol/kg (0.003, 0.03) and 0.08 μmol/kg (0.05, 0.22) in the placebo group compared to 0.79 μmol/kg (0.52, 3.66) and 1.02 μmol/kg (0.65, 3.15), respectively, in the isoflavone group (Table 2). All placebo vs. treatment differences were statistically significant (p<0.001). Aglycones were not detected.
Table 2.
Isoflavone concentrations in serum and prostate tissue for the treatment group
| Participant ID | Genistein | Daidzein | ||||
|---|---|---|---|---|---|---|
| Tissue μmol/kg | Serum μmol/L | Tissue/Serum Ratio | Tissue μmol/kg | Serum μmol/L | Tissue/Serum Ratio | |
| 515 | 4.21 | 0.69 | 6.09 | 5.08 | 0.33 | 15.61 |
| 517 | 0.56 | 0.31 | 1.82 | 0.61 | 0.09 | 6.86 |
| 518 | 3.66 | 0.87 | 4.20 | 3.15 | 0.35 | 8.99 |
| 522 | 2.15 | 0.52 | 4.12 | 2.11 | 0.22 | 9.42 |
| 532 | 0.33 | 0.22 | 1.52 | 0.59 | 0.06 | 9.89 |
| 533 | 0.70 | 0.11 | 6.46 | 0.66 | 0.04 | 15.10 |
| 538 | 9.15 | 0.94 | 9.77 | 10.20 | 1.44 | 7.07 |
| 540 | 2.30 | 0.79 | 2.91 | 1.02 | 0.27 | 3.77 |
| 555 | 0.79 | 0.31 | 2.55 | 1.29 | 0.21 | 6.25 |
| 557 | 0.52 | 0.12 | 4.49 | 0.79 | 0.06 | 13.50 |
| 560 | 0.14 | 0.11 | 1.35 | 0.65 | 0.05 | 13.97 |
| Mean ± SD | 2.23 ±2.68 | 0.45 ±0.32 | 4.1± 2.6 | 2.38 ±2.95 | 0.28± 0.40 | 10.0 ± 4.0 |
| Median (25th, 75th) | 0.79 (0.52, 3.66) † | 0.31 (0.11, 0.79)† | 1.02 (0.65, 3.15)† | 0.21 (0.06, 0.33)† | ||
| P=0.001 ‡ | P=0.003 ‡ | |||||
The tissue/serumratio for genistein is significantly lower than that for daidzein, p=0.003(matched pairs t-test).
The tissue and serum concentrations of genistein and daidzein were notibuted, so values for the median and the 25th and 75th percentile are presented in addition to the means. The tissue/serum ratios were normally distributed, and medians are not presented for these values.
Testing for statistical significance in the difference between tissue and serum levels was conducted by matched pairs t-test.
Serum concentrations of isoflavones
The serum concentration (median; 25th, 75th percentiles) of total isoflavones was 0.01 μmol/L (0.005, 0.01) in the placebo compared to 0.75 μmol/L (0.17, 1.21) in the isoflavone group. For genistein and daidzein the serum concentrations were 0.002 μmol/L (0, 0.003) and 0.008 μmol/L (0.004, 0.01) in the placebo group compared to 0.31 μmol/L (0.11, 0.79) and 0.21 μmol/L (0.06, 0.33), respectively, in the isoflavone group (Table 2). All placebo vs. treatment differences were statistically significant (p≤0.003).
Two of the 11 participants in the isoflavone group had serum concentrations of equol >50 nmol/L (participatent ID#s 540 and 555). These numbers were considered too few for further analyses of equol producers as a separate group.
Ratio between tissue and serum concentration in treatment group
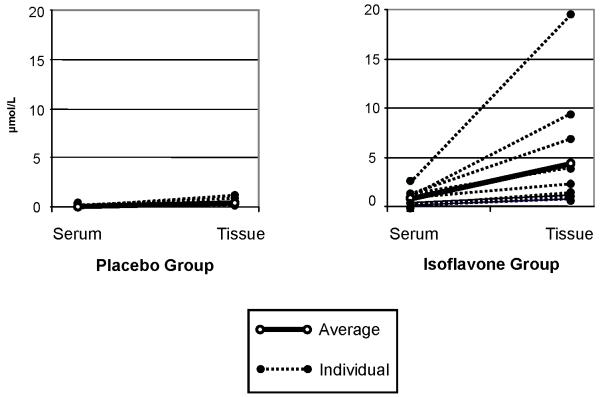
The ratio of total isoflavone concentrations between tissue (μmol/kg) and serum (μmol/L) among the isoflavone group (mean ± SD) was 5.7 ± 2.5. Individual patient data are shown in Figure 1. Tissue vs. serum ratio for genistein 4.1 ± 2.6 was lower than for daidzein 10.0 ± 4.0 (p=0.003) (Table 2).
Figure 1.
Total serum and prostatic tissue isoflavone concentration for the 1 placebo and the treatment group
Correlations between tissue and serum concentration in treatment group
The tissue isoflavone concentrations were highly correlated to the serum isoflavone concentrations. For genistein and daidzein in the treatment group the correlation was r = 0.83 and r = 0.95, respectively.
Discussion
The present study took advantage of access to human prostate tissue collected during routine prostatectomy procedures to examine and contrast tissue and serum concentrations of isoflavones that had been administered by dietary supplementation for at least two weeks prior to surgery. There was a ∼6-fold higher total isoflavone concentration in the tissue compared to serum levels sampled at the time of tissue harvesting. . Further examination of specific types of isoflavones indicated that the tissue vs. serum difference was smaller for genistein (4-fold) than for daidzein (10-fold).
The two primary soy isoflavones - genistein and daidzein - have been extensively investigated for their anticancer effects in vitro and in animal studies. In models pertaining to PCA, genistein inhibits receptor tyrosine kinases, nuclear factor kappa B (NF-kB) and vitamin D-24-hydroxylase thus induces apoptosis (10, 13) and enhances calcitriol action to inhibit proliferation of PCA cells (29, 37). Genistein activates tumor suppressor genes (12, 15), inhibits metastasis (13), modulates androgen-responsive gene expression (14), and down-regulates prostate-specific antigen (PSA) (9) and the androgen receptor (7).
Daidzein exhibits similar effects on apoptosis and cell cycle (17), but has been reported to be less effective (16). However, daidzein, unlike genistein, is metabolized to equol with a longer half-life than genistein and daidzein (8) which has been shown to inhibit neoplastic transformation (11).
The anti-carcinogenic effects of isoflavones are importantly dose dependent. In cell culture studies, concentrations of 10 μmol/L or higher were determined to be anti-proliferative (27-29). A review of 19 bioavailability and 17 intervention studies with orally administered isoflavones ranging in dose from 37-128 mg/day reported peak serum levels of approximately 2 μmol/L that were achieved after ∼6-8 hours. Many human studies examining serum concentrations have reported levels in the 1 μmol/L range (38-41). The relatively high doses used in cell culture and animal model investigations raise questions as to whether biologically active isoflavone levels can be achieved in humans by oral ingestion. Perhaps more important is whether assessment of blood levels of isoflavones is a relevant surrogate measure of tissue levels, particularly in the prostate. Tissue levels were not determined in any of the studies cited above. In our study the administration of 82 mg total isoflavones per day resulted in a fasting total isoflavone serum level of 0.7 μmol/L with a corresponding tissue level of 2.3 μmol/L. The dose of 82 mg/day used in the study can be realistically achieved by natural dietary sources (42).
Due to the greater accessibility to prostatic fluid relative to prostate tissue, and the less invasive sampling procedure, several studies have primarily observed the concentration of isoflavone within the prostatic fluid, with the assumption that this would correlate with tissue levels. Morton et al. observed that seminal fluid daidzein concentrations were twice that of serum (43) and Hedlund et al., showed that prostate fluid taken from participants after isoflavone supplementation showed sufficient concentrations of genistein and daidzein to inhibit the growth of cultured prostatic epithelial cells (27). However, the indirect assessment of the prostatic concentration by sampling the secreted glandular fluid may not reflect cellular levels and we are aware of no published work that has evaluated the strength of the correlation of isoflavone concentration between the prostatic tissue and seminal fluid. Direct measurement of prostate tissue levels of isoflavones, such as were obtained in our study, are more likely to be physiologically relevant than those obtained from prostatic fluid.
Limited data are available on isoflavone concentrations measured directly in prostate tissues. In agreement with our findings Farhan et al. reported a 10-fold increase in mouse prostate genistein concentrations compared to serum after only one oral dose of 250 mg of genistein (31). Similarly, Hong et al. were the first to measure isoflavones in human prostatic tissue and reported consistently higher isoflavone concentrations in the prostate than in serum (34). In that study, tissues were obtained from several sources including cystoprostatectomy and transurethral resection, all specimens were benign prostatic tissue, and subjects were not supplemented with isoflavones in their diet. Brössner et al. compared tissue concentrations of genistein in 63 men with benign prostatic hyperplasia (BPH) and 31 with PCA on a traditional Western diet (32). The study found lower genistein concentrations in the PCA group than the BPH group, but neither assessed the serum level of the participants nor were subjects supplemented with isoflavones.
Ranniko et al. were the first to conduct a randomized placebo-controlled study investigating the effect of oral isoflavone supplementation on serum and tissue levels in a cohort of 40 men undergoing prostatectomy for cancer. After 2 weeks of 240 mg clover phytoestrogen supplementation, a two-fold higher tissue than serum concentration was reported (35). In comparison to our study, despite the lower daily dosage of 82 mg for two weeks in our study, a slightly lower serum level was reached, but the tissue vs. serum ratio was ∼6-fold. Among the limited number of studies in this area, only Guy et al. reported the contrary result with tissue concentrations observed to be lower than serum isoflavone concentration in 12 participants. The study population, however, consisted of men with BPH rather than PCA and used prostatic tissue chips. In our study, tissue levels were determined using sections from whole prostatic specimens from PCA patients.
The higher tissue/serum ratio of daidzein vs. genistein is noteworthy and indicates that despite the faster urinary excretion of daidzein vs. genistein(44) the former is better concentrated into prostatic tissue than the latter. The predominant presence of the isoflavones as glucuronide and sulfate conjugates in prostate reflects the situation in the circulation but due to the low number of subjects investigated this needs to be confirmed in future larger studies.
Conclusions
Based on our finding that the local isoflavone concentration within the target tissue was significantly higher than the serum level we conclude that prostatic tissue is able to accumulate potentially anticancerous isoflavones. Further understanding of the mechanism involved in the ability to accumulate isoflavones and the distribution and fate of isoflavones among the benign and malignant prostatic tissue may provide additional insights into the dietary link of soy isoflavones to chemoprevention.
Supplementary Material
Acknowledgements
The authors acknowledge the work of Laurie Custer for the skilful performance of tissue extractions and LCMS analyses. We also thank Michelle Ferrari, RN, for coordinating clinical data and specimens, and Joel Nicholus, MS, for his overall coordination of the study.
Grant Support:
This investigation was supported by NIH grants AT00486 and P30 CA71789, by Human Health Service grant M01-RR00070 and Special Instrumentation Grant S10-RR20890 from the National Center for Research Resources, National Institutes of Health.
REFERENCES
- 1.AmericanCancerSociety . Cancer Facts & Figures 2007. American Cancer Society; 2007. [Google Scholar]
- 2.Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger RS., Jr. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9(8):795–804. [PubMed] [Google Scholar]
- 3.Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S. Soy isoflavone consumption is not associated with increased risk of advanced prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2169. doi: 10.1158/1055-9965.EPI-07-0414. [DOI] [PubMed] [Google Scholar]
- 4.Yan L, Spitznagel EL. Meta-analysis of soy food and risk of prostate cancer in men. Int J Cancer. 2005;117(4):667–669. doi: 10.1002/ijc.21266. [DOI] [PubMed] [Google Scholar]
- 5.Messina MJ. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev. 2003;61(4):117–131. doi: 10.1301/nr.2003.apr.117-131. [DOI] [PubMed] [Google Scholar]
- 6.Marks LS, Kojima M, Demarzo A, Heber D, Bostwick DG, Qian J, Dorey FJ, Veltri RW, Mohler JL, Partin AW. Prostate cancer in native Japanese and Japanese-American men: effects of dietary differences on prostatic tissue. Urology. 2004;64(4):765–771. doi: 10.1016/j.urology.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Bektic J, Berger AP, Pfeil K, Dobler G, Bartsch G, Klocker H. Androgen receptor regulation by physiological concentrations of the isoflavonoid genistein in androgen-dependent LNCaP cells is mediated by estrogen receptor beta. Eur Urol. 2004;45(2):245–251. doi: 10.1016/j.eururo.2003.09.001. discussion 251. [DOI] [PubMed] [Google Scholar]
- 8.Chang YC, Nair MG, Nitiss JL. Metabolites of daidzein and genistein and their biological activities. J Nat Prod. 1995;58(12):1901–1905. doi: 10.1021/np50126a016. [DOI] [PubMed] [Google Scholar]
- 9.Davis JN, Kucuk O, Sarkar FH. Expression of prostate-specific antigen is transcriptionally regulated by genistein in prostate cancer cells. Mol Carcinog. 2002;34(2):91–101. doi: 10.1002/mc.10053. [DOI] [PubMed] [Google Scholar]
- 10.Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer. 1999;35(2):167–174. doi: 10.1207/S15327914NC352_11. [DOI] [PubMed] [Google Scholar]
- 11.Kang NJ, Lee KW, Rogozin EA, Cho YY, Heo YS, Bode AM, Lee HJ, Dong Z. Equol, a metabolite of the soybean isoflavone daidzein, inhibits neoplastic cell transformation by targeting the MEK/ERK/p90RSK/activator protein-1 pathway. J Biol Chem. 2007;282(45):32856–32866. doi: 10.1074/jbc.M701459200. [DOI] [PubMed] [Google Scholar]
- 12.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, Majid S, Igawa M, Dahiya R. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int J Cancer. 2008 doi: 10.1002/ijc.23590. [DOI] [PubMed] [Google Scholar]
- 13.Lakshman M, Xu L, Ananthanarayanan V, Cooper J, Takimoto CH, Helenowski I, Pelling JC, Bergan RC. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer research. 2008;68(6):2024–2032. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- 14.Lazarevic B, Karlsen SJ, Saatcioglu F. Genistein differentially modulates androgen-responsive gene expression and activates JNK in LNCaP cells. Oncol Rep. 2008;19(5):1231–1235. [PubMed] [Google Scholar]
- 15.Majid S, Kikuno N, Nelles J, Noonan E, Tanaka Y, Kawamoto K, Hirata H, Li LC, Zhao H, Okino ST, Place RF, Pookot D, Dahiya R. Genistein induces the p21WAF1/CIP1 and p16INK4a tumor suppressor genes in prostate cancer cells by epigenetic mechanisms involving active chromatin modification. Cancer research. 2008;68(8):2736–2744. doi: 10.1158/0008-5472.CAN-07-2290. [DOI] [PubMed] [Google Scholar]
- 16.Verma SP, Goldin BR. Effect of soy-derived isoflavonoids on the induced growth of MCF-7 cells by estrogenic environmental chemicals. Nutr Cancer. 1998;30(3):232–239. doi: 10.1080/01635589809514669. [DOI] [PubMed] [Google Scholar]
- 17.Lo FH, Mak NK, Leung KN. Studies on the anti-tumor activities of the soy isoflavone daidzein on murine neuroblastoma cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2007;61(9):591–595. doi: 10.1016/j.biopha.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Lund TD, Munson DJ, Haldy ME, Setchell KD, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod. 2004;70(4):1188–1195. doi: 10.1095/biolreprod.103.023713. [DOI] [PubMed] [Google Scholar]
- 19.Joniau S, Goeman L, Roskams T, Lerut E, Oyen R, Van Poppel H. Effect of nutritional supplement challenge in patients with isolated high-grade prostatic intraepithelial neoplasia. Urology. 2007;69(6):1102–1106. doi: 10.1016/j.urology.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton-Reeves JM, Rebello SA, Thomas W, Kurzer MS, Slaton JW. Effects of soy protein isolate consumption on prostate cancer biomarkers in men with HGPIN, ASAP, and low-grade prostate cancer. Nutr Cancer. 2008;60(1):7–13. doi: 10.1080/01635580701586770. [DOI] [PubMed] [Google Scholar]
- 21.Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, Doerge D, Fontana J, Chinni S, Davis J, Forman J, Wood DP, Kucuk O. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47(2):111–117. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 22.Spentzos D, Mantzoros C, Regan MM, Morrissey ME, Duggan S, Flickner-Garvey S, McCormick H, DeWolf W, Balk S, Bubley GJ. Minimal effect of a low-fat/high soy diet for asymptomatic, hormonally naive prostate cancer patients. Clin Cancer Res. 2003;9(9):3282–3287. [PubMed] [Google Scholar]
- 23.Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, Forman JD, Cher ML, Powell I, Pontes JE, Kucuk O. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59(1):1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 24.Perabo FG, Von Low EC, Ellinger J, von Rucker A, Muller SC, Bastian PJ. Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2008;11(1):6–12. doi: 10.1038/sj.pcan.4501000. [DOI] [PubMed] [Google Scholar]
- 25.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 26.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81(1 Suppl):243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 27.Hedlund TE, Maroni PD, Ferucci PG, Dayton R, Barnes S, Jones K, Moore R, Ogden LG, Wahala K, Sackett HM, Gray KJ. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men. J Nutr. 2005;135(6):1400–1406. doi: 10.1093/jn/135.6.1400. [DOI] [PubMed] [Google Scholar]
- 28.Zava DT, Duwe G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr Cancer. 1997;27(1):31–40. doi: 10.1080/01635589709514498. [DOI] [PubMed] [Google Scholar]
- 29.Swami S, Krishnan AV, Peehl DM, Feldman D. Genistein potentiates the growth inhibitory effects of 1,25-dihydroxyvitamin D3 in DU145 human prostate cancer cells: role of the direct inhibition of CYP24 enzyme activity. Mol Cell Endocrinol. 2005;241(1-2):49–61. doi: 10.1016/j.mce.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007;57(1):1–10. doi: 10.1080/01635580701267677. [DOI] [PubMed] [Google Scholar]
- 31.Farhan H, Wahala K, Adlercreutz H, Cross HS. Isoflavonoids inhibit catabolism of vitamin D in prostate cancer cells. Journal of chromatography. 2002;777(1-2):261–268. doi: 10.1016/s1570-0232(02)00081-8. [DOI] [PubMed] [Google Scholar]
- 32.Brossner C, Petritsch K, Fink K, Auprich M, Madersbacher S, Adlercreutz H, Rehak P, Petritsch P. Phytoestrogen tissue levels in benign prostatic hyperplasia and prostate cancer and their association with prostatic diseases. Urology. 2004;64(4):707–711. doi: 10.1016/j.urology.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 33.Guy L, Vedrine N, Urpi-Sarda M, Gil-Izquierdo A, Al-Maharik N, Boiteux JP, Scalbert A, Remesy C, Botting NP, Manach C. Orally administered isoflavones are present as glucuronides in the human prostate. Nutr Cancer. 2008;60(4):461–468. doi: 10.1080/01635580801911761. [DOI] [PubMed] [Google Scholar]
- 34.Hong SJ, Kim SI, Kwon SM, Lee JR, Chung BC. Comparative study of concentration of isoflavones and lignans in plasma and prostatic tissues of normal control and benign prostatic hyperplasia. Yonsei medical journal. 2002;43(2):236–241. doi: 10.3349/ymj.2002.43.2.236. [DOI] [PubMed] [Google Scholar]
- 35.Rannikko A, Petas A, Rannikko S, Adlercreutz H. Plasma and prostate phytoestrogen concentrations in prostate cancer patients after oral phytoestogen supplementation. The Prostate. 2006;66(1):82–87. doi: 10.1002/pros.20315. [DOI] [PubMed] [Google Scholar]
- 36.Gardner CD, Chatterjee LM, Franke AA. Effects of isoflavone supplements vs. soy foods on blood concentrations of genistein and daidzein in adults. J Nutr Biochem. 2008 doi: 10.1016/j.jnutbio.2008.02.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swami S, Krishnan AV, Moreno J, Bhattacharyya RB, Peehl DM, Feldman D. Calcitriol and genistein actions to inhibit the prostaglandin pathway: potential combination therapy to treat prostate cancer. J Nutr. 2007;137(1 Suppl):205S–210S. doi: 10.1093/jn/137.1.205S. [DOI] [PubMed] [Google Scholar]
- 38.Heald CL, Ritchie MR, Bolton-Smith C, Morton MS, Alexander FE. Phyto-oestrogens and risk of prostate cancer in Scottish men. Br J Nutr. 2007;98(2):388–396. doi: 10.1017/S0007114507700703. [DOI] [PubMed] [Google Scholar]
- 39.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130(7):1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe S, Yamaguchi M, Sobue T, Takahashi T, Miura T, Arai Y, Mazur W, Wahala K, Adlercreutz H. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako) J Nutr. 1998;128(10):1710–1715. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- 41.Wiseman H, Casey K, Bowey EA, Duffy R, Davies M, Rowland IR, Lloyd AS, Murray A, Thompson R, Clarke DB. Influence of 10 wk of soy consumption on plasma concentrations and excretion of isoflavonoids and on gut microflora metabolism in healthy adults. Am J Clin Nutr. 2004;80(3):692–699. doi: 10.1093/ajcn/80.3.692. [DOI] [PubMed] [Google Scholar]
- 42.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55(1):1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 43.Morton MS, Chan PS, Cheng C, Blacklock N, Matos-Ferreira A, Abranches-Monteiro L, Correia R, Lloyd S, Griffiths K. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. The Prostate. 1997;32(2):122–128. doi: 10.1002/(sici)1097-0045(19970701)32:2<122::aid-pros7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 44.Franke AA, Custer LJ, Hundahl SA. Determinants for urinary and plasma isoflavones in humans after soy intake. Nutr Cancer. 2004;50(2):141–154. doi: 10.1207/s15327914nc5002_3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.