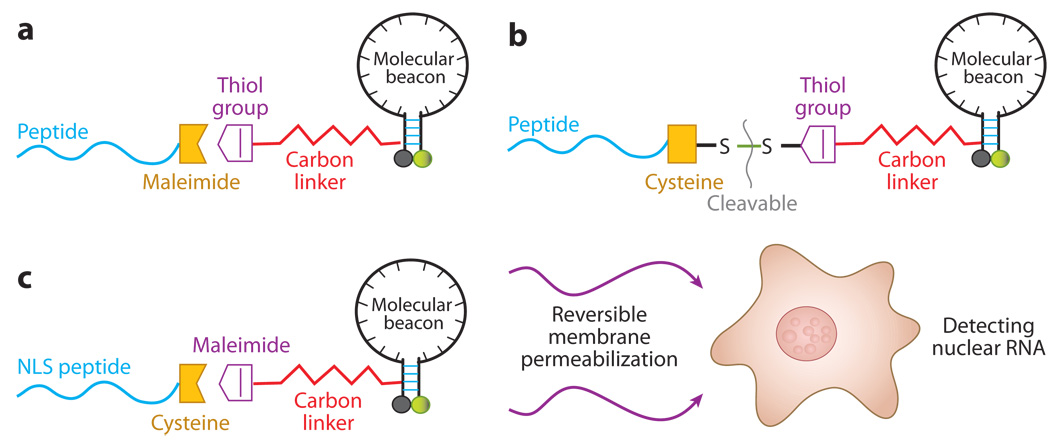
Figure 8.
A schematic of peptide-linked molecular beacons. (a) A peptide-linked molecular beacon using the thiol-maleimide linkage in which the quencher arm of the molecular beacon stem is modified by adding a thiol group, which can react with a maleimide group placed to the C terminus of the peptide to form a direct, stable linkage. (b) A peptide-linked molecular beacon with a cleavable disulfide bridge in which the peptide is modified by adding a cysteine residue at the C terminus, which forms a disulfide bridge with the thiol-modified molecular beacon. This disulfide bridge design allows the peptide to be cleaved from the molecular beacon by the reducing environment of the cytoplasm. (c) A schematic illustration of the design of a peptide-linked molecular beacon and its delivery into cell nucleus. The NLS peptide is covalently linked to the molecular beacon using a modified nucleotide in its quencher arm. The NLS-linked molecular beacons are delivered into the cytoplasm first using streptolysin O (SLO), and the NLS peptide actively transports the probes into the nucleus of a living cell.