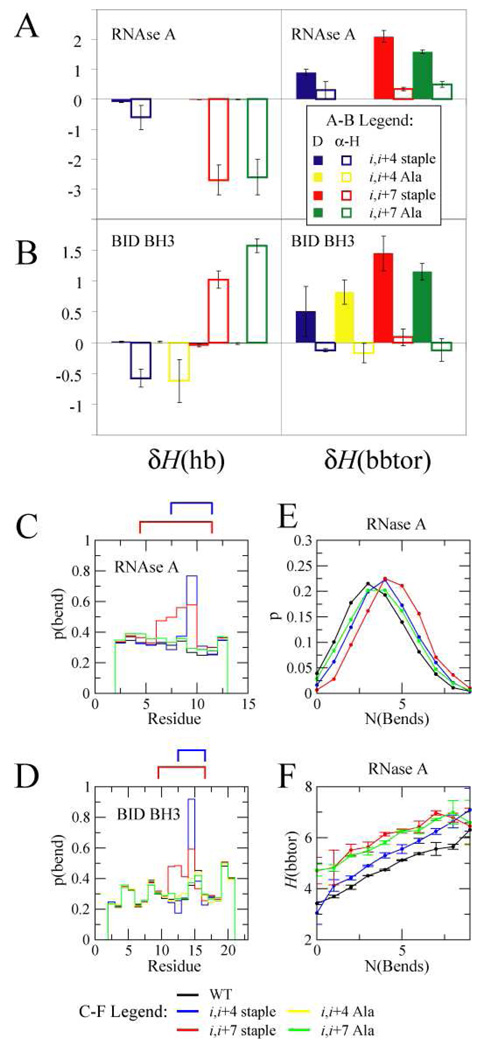
Figure 4.
RNAse A (A) and BID BH3 (B) changes in hydrogen bonding δH(hb) and sequence-dependent backbone torsions δH(bbtor) of denatured (D) and helical (α-H) states of stapled and Ala mutant peptides relative to the corresponding WT peptide. Probability that a residue is classified as a bend for RNAse A (C) and BID BH3 (D). A cartoon depicting the staple is above the plot. E: Probability of finding conformations with a certain number of “bend” residues in the denatured state for RNAse A peptides. F: The average backbone triplet energy H(bbtor) of conformations in the denatured state versus number of “bend” residues. RNAse A and BID BH3 simulations were carried out at T=0.78 and T=0.70, respectively. Colors of peptides in C, D, E, and F are as follows: WT (black), i,i+4 stapled (blue), i,i+7 stapled (red), i,i+4 Ala (yellow), i,i+7 stapled (green).