Table 1.
Optimization of Aldol Reactiona
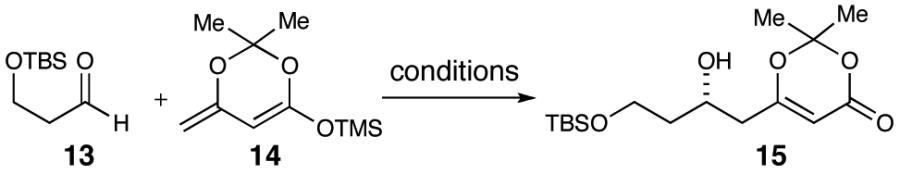 | |||||
|---|---|---|---|---|---|
| entry | solvent | R-BINOL (mol %) | additive | yield (%)b | ee (%)c |
| 1 | THF | 8 | 4 Å MS | 50 | 73d,e |
| 2 | THF | 8 | 4 Å MS | 53 | 71d |
| 3 | THF | 10 | 4 Å MS | 60 | 78d,e |
| 4 | toluene | 10 | 4 Å MS | 30 | 71 |
| 5 | CH2Cl2 | 10 | 4 Å MS | — | — |
| 6 | THF | 10 | 3 Å MSf | 65 | 68 |
| 7 | THF | 10 | 3 Å MS | 66 | 63 |
| 8 | THF | 10 | 3 Å MSf,g | 65 | 81 |
| 9 | THF | 10 | 4 Å MSg | 57 | 74 |
| 10 | THF | 15 | 4 Å MSg | 66 | 80 |
| 11 | THF | 10 | 4 Å MS | 63 | 88 |
| 12 | THF | 15 | 4 Å MS | 64 | 60 |
Reactions performed under N2 at 0.17 M (precomplexation concentration 0.53 M) with Ti(i-OPr)4 and ligand after 60 min, reaction cooled to -78 °C followed by addition of 13 and 14.
Isolated yields after column chromatography.
Determined by chiral HPLC.
precomplexation concentration 0.21 M.
Slowly warmed to 23 °C.
3 Å MS pellets.
Dried in a reduced pressure oven at 145 °C for 2 days.
