Standard lipid tests measure the cholesterol or triglyceride content of lipoproteins, expressed as mg/dL (or mmol/L) of cholesterol or triglyceride. A standard lipid panel includes total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides. These lipids are carried within lipoprotein particles that are heterogeneous in size, density, charge, core lipid composition, specific apolipoproteins, and function.1 A variety of lipoprotein assays have been developed that subfractionate lipoprotein particles according to some of these properties such as size, density, or charge.2 These lipoprotein assays have been proposed for improving assessment of risk of cardiovascular disease (CVD) and for guiding lipid lowering therapies. They are mostly used today in specialized lipid clinics or research studies and their general utility for clinical practice is not well delineated.3 In this review, I first provide a summary of current guideline statements that address this topic. Then I give a brief overview of the different laboratory methods available for clinical use and discuss the literature evaluating these methods. Finally, I discuss current limitations to the widespread clinical application of these methods.
Guideline Recommendations
The Third Adult Treatment Panel (ATP III) of the National Cholesterol Education Program4 and the American Heart Association5 recommend measuring a standard lipid panel in adults and targeting lipid lowering therapy based on levels of LDL cholesterol, and non-HDL cholesterol in subjects with hypertriglyceridemia. European and Canadian guidelines recommend measuring standard lipids, while also emphasizing the total/HDL cholesterol ratio in risk assessment.6,7 Current guidelines do not recommend routine use of advanced lipoprotein tests, mainly because it is unclear how much incremental prognostic information is provided by these tests beyond what is available from a standard lipid panel.4,6,7
Recently, an international panel proposed that cardiovascular risk may be more closely related to atherogenic lipoprotein particle number than LDL cholesterol (the atherogenic lipoprotein particle paradigm).8 This paradigm posits that lipid concentrations in lipoproteins do not equal the concentrations of the lipoproteins themselves, and that measuring the concentration of lipoproteins may be superior to measuring their lipid concentrations for estimating CVD risk and determining the adequacy of statin therapy.8 A subsequent consensus statement endorsed by the American Diabetes Association (ADA) and the American College of Cardiology (ACC) advocated measuring atherogenic particle concentration either as apolipoprotein B (apoB) or LDL particle concentration in subjects at high risk for cardiometabolic disorders for assessing CVD risk and guiding therapy, in conjunction with using LDL and non-HDL cholesterol.9
Laboratory Methods
A thorough discussion of the various advanced lipoprotein tests is beyond the scope of this review,10 but a brief summary of the common tests is provided below. Several tests use proprietary techniques that have not been published. The number and nomenclature of lipoprotein subfractions are not uniform across the different techniques and have not been standardized, making it difficult to compare results from various tests. The National Academy of Clinical Biochemistry recently called for standardization of the technologies used to determine lipoprotein subfractions.11 Several of these tests can only be performed at the company that markets the test, limiting the ability to obtain independent information on test performance.
Apolipoproteins
Apolipoproteins are measured in routine clinical laboratories using immunonephelometric or immunoturbidimetric assays. Importantly, international standards have been developed for apolipoprotein B100 (apoB) and A-1.11 ApoB reflects the number of potentially atherogenic lipoprotein particles, since each particle of very-low-density lipoprotein (VLDL), β-VLDL, intermediate-density lipoprotein (IDL), LDL, and lipoprotein (a) particle carries on its surface one apoB100 protein. Most of plasma apoB is found in LDL particles.12 HDL particles do not carry apoB, but instead carry apolipoprotein A-1 (apoA-1).12 However, apoA-1 does not correspond directly to the concentration of HDL particles in the one-to-one fashion seen for apoB100 and LDL particles, since an HDL particle may carry more than one apoA-1 protein.
Gradient Gel Electrophoresis (GGE) available from Berkeley HeartLab, Inc. (California)
This method determines the distribution of LDL size phenotype by proprietary segmented polyacrylamide gradient gels, which separate lipoproteins in a gradient gel based on their size and, to a lesser extent, their charge.13 Pattern A corresponds to large LDL particles, B to small, dense LDL particles, and AB to an intermediate phenotype. This method gives the relative, or predominant, distribution of lipoprotein particles as determined by the predominant peak particle size. ApoB or ultra-apoB (LDL apoB) is measured by immunoassay for additional cost per request.
Density Gradient Ultracentrifugation available as the VAP II test from Atherotec, Inc. (Alabama)
This method measures the relative distribution of cholesterol within various lipoprotein subfractions, quantifying the cholesterol content of VLDL, IDL, LDL, lipoprotein (a), and HDL subclasses.14 VAP also determines the predominant LDL size distribution (eg. A, AB or B phenotype) but does not provide concentrations of the lipoprotein particles themselves. ApoB is provided at no additional cost, although it is not measured directly.
Nuclear Magnetic Resonance (NMR) Spectroscopy available from LipoScience, Inc. (North Carolina)
This technique is based on the concept that each lipoprotein particle in plasma of a given size has its own characteristic lipid methyl group NMR signal. Particle concentrations of lipoprotein subfractions of different size are obtained from the measured amplitudes of their lipid methyl group NMR signals. Lipoprotein particle sizes are then derived from the sum of the diameter of each subclass multiplied by its relative mass percentage based on the amplitude of its methyl NMR signal. NMR LipoProfile-II simultaneously quantifies lipoprotein concentrations of VLDL, IDL, LDL, and HDL particles and their subfractions, expressed each as a lipoprotein particle concentration (# particles/L) or as an average particle size for each of VLDL, LDL, and HDL.15–18
Ion-Mobility Analysis available from Quest Diagnostics, Inc. (California)
This is a newly developed method that measures both the size and concentrations of lipoprotein particle subclasses based on gas-phase differential electrical mobility.19
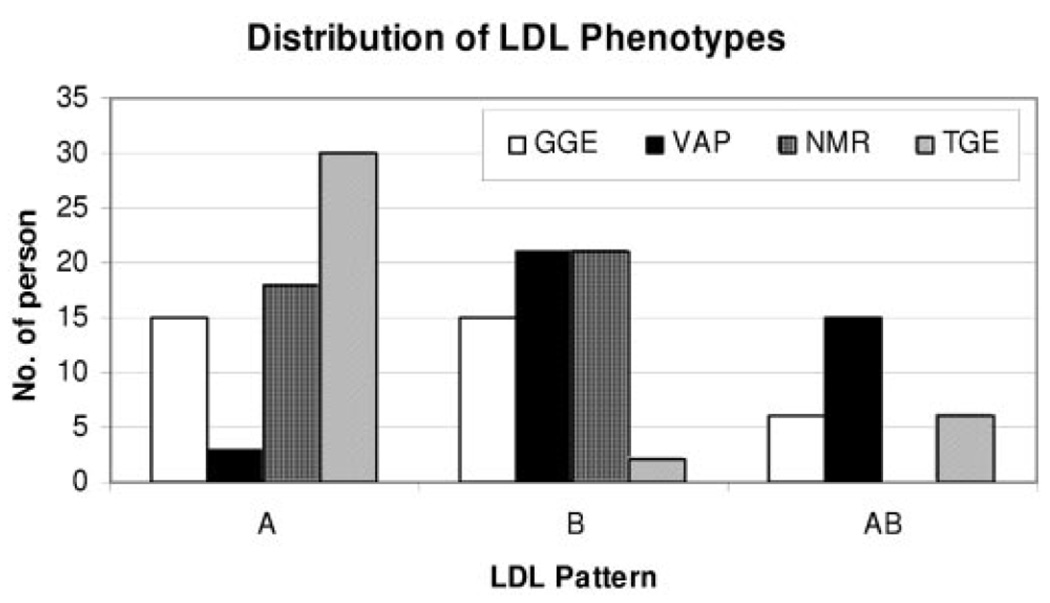
Direct comparisons of these techniques is limited. The correlation for LDL size between NMR and GGE was 0.86 in a small study of men.20 In another study (N=324 individuals), LDL size by GGE and NMR was only moderately correlated (Spearman correlation 0.4) and the chance-adjusted Kappa statistic was moderate (0.3).21 A more recent study by Ensign et al22 (N=40 individuals) found the agreement between GGE and NMR to be 70%. However, when results were compared across 4 methods that are used to determine LDL size, complete agreement among the 4 methods examined (GGE, NMR, VAP and tube gel electrophoresis [TGE]) for LDL size phenotype was only 8% (Figure 1). This highlights the important need for standardization if these measurements are to be more widely used in clinical practice, especially given the fact that the methods use different principles for subfractionation of lipoproteins.23
Figure 1.
Distribution of LDL phenotypes in 40 individuals showed poor agreement for LDL size measurement among 4 commonly used advanced lipoprotein tests.22
What are advanced lipoprotein tests often used for in clinical practice?
1. CVD risk assessment
To enhance CVD risk assessment, especially in individuals with low or normal LDL cholesterol, by detecting: a) the presence of higher concentrations of atherogenic lipoprotein particle concentrations (eg. apoB or NMR LDL particle concentration), or b) the presence of small dense LDL particles, which are commonly believed to be more atherogenic than larger LDL particles. There is emerging, and conflicting, data about the potential atherogenicity of the various HDL and VLDL particle subfractions, although less is known compared with LDL subfractions.24
2. Targets for lipid lowering therapy
To detect “residual risk” in patients who are already treated with lipid lowering therapy, often with statins.9 Advocates of advanced lipoprotein testing point out that ~70% of the patients who otherwise would have had CVD events still experience CVD events, despite LDL-C lowering on statin therapy. This is often referred to as “residual risk”
1. CVD Risk Assessment
Currently, the best validated measure of CVD risk obtained from advanced lipoprotein testing is the concentration of atherogenic particles, such as apoB or NMR LDL particle concentration.9 First, I discuss how apoB and NMR LDL particle concentration compare with non-HDL cholesterol or the total/HDL cholesterol ratio. Second, I discuss how smaller LDL size may not be an independent risk factor for CVD but instead a surrogate marker for a higher concentration of atherogenic particles.
1.A. Is apoB (or LDL particle concentration) superior to non-HDL cholesterol or the total/HDL cholesterol ratio for CVD risk assessment?
Outside of standard lipids, apoB is the most widely studied lipoprotein parameter, in both primary and secondary prevention populations and statin trials.8,12,25 Most of these studies, but not all,26 found that apoB was more closely associated with CVD risk than LDL cholesterol, as summarized elsewhere.8,27 Similarly, although in fewer studies than apoB, CVD risk is more closely associated with NMR LDL particle concentration than LDL cholesterol.20,28–30 What remains controversial is whether apoB (or LDL particle concentration) is superior to non-HDL cholesterol or total/HDL cholesterol ratio for CVD risk prediction, and whether potentially small improvements in risk prediction are offset by potentially higher healthcare costs from additional tests.31–36
Two large studies (Apolipoprotein-related MOrtality RISk [AMORIS] and INTERHEART) have contributed valuable insight to risk factors worldwide, and concluded that apoB was superior to standard lipids.31,37,38 However, these studies have study design limitations. AMORIS used lipids that were not measured by recommended standards, and did not have full adjustment for other risk factors.37 INTERHEART, an international case-control study, measured lipids in the setting of acute hospitalization or myocardial infarction, which may affect the association of lipids and apolipoproteins with disease. For example, INTERHEART found that higher triglycerides were associated with statistically significantly lower risk of myocardial infarction,38 a rather unusual finding that stands in contrast to most studies that have found increased risk of CVD with higher triglycerides. INTERHEART also did not have full adjustment for other risk factors.
Both apoB and LDL particle concentration correlate with LDL cholesterol, but they correlate even more with non-HDL cholesterol and the total/HDL cholesterol ratio (correlation coefficient ~0.7–0.8).32,33,39 Measures that are highly correlated, as are apoB (or LDL particle concentration) and non-HDL cholesterol or total/HDL cholesterol, make the results obtained from standard regression models unstable (unreliable) because of co-linearity. A better way to assess the clinical utility of a lipoprotein test for CVD risk prediction, in particular for highly correlated measures, is the degree to which it enhances the accuracy of existing cardiovascular risk assessment tools.
From a clinical and public health standpoint, the key issue is whether the use of a test results in more appropriate choice of therapy and improves patient outcomes, when used either in addition to or instead of standard risk factors. The reclassification of individuals, in particular those with intermediate risk, to a higher or lower risk category could have important implications for preventive pharmacotherapy in these patients.
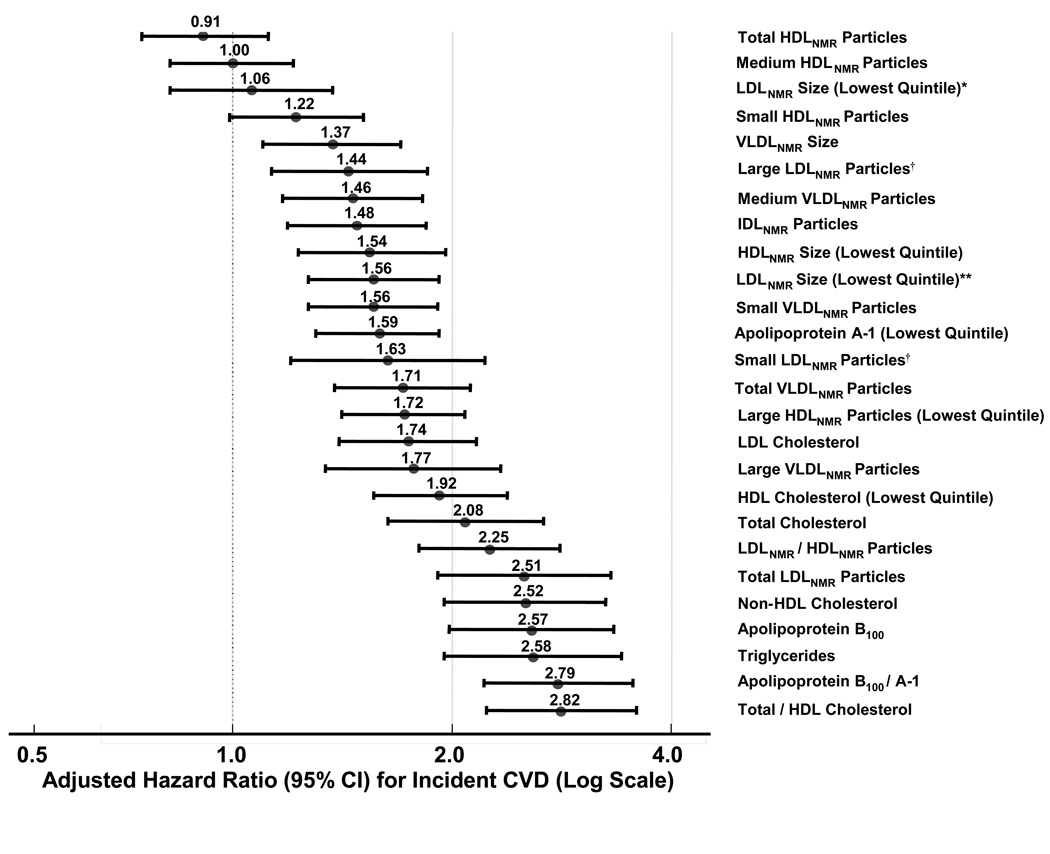
The net reclassification index (NRI) is a quantitative measure of model fit that compares the proportion of individuals moving up or down in risk categories with the use of a biomarker, and has been proposed to assess whether a biomarker adds information to traditional risk factors.40 The NRI compares shifts in reclassified categories by observed outcome, and is penalized by incorrect classification.40 To date, two studies have evaluated the predictive performance of apoB or NMR LDL particle concentration for risk reclassification of asymptomatic individuals compared with standard lipids. In the Framingham study, there was little additional risk information obtained from apoB or apoB/A-1 ratio compared with the total/HDL cholesterol ratio.33 The difference in the NRI for apoB/A-1 compared with total/HDL cholesterol was very small (0.1%) and statistically non-significant.33 In the Women’s Health Study, both apoB and NMR LDL particle concentration were significantly associated with CVD over an 11-year follow-up of 27,000 women (Figure 2), with a magnitude of association comparable to total/HDL cholesterol or non-HDL cholesterol. However, the NRI for adding either apoB or NMR LDL particle concentration to the total/HDL cholesterol ratio was <2%.34
Figure 2.
Association of standard lipids, NMR lipoproteins, and immunoassay apolipoproteins with incident CVD in 27,673 initially healthy women, Women’s Health Study.34
*LDLNMR size adjusted for non-lipid risk factors and total LDLNMR particle concentration (number).
LDLNMR size adjusted only for non-lipid risk factors.
†Large and small LDLNMR particles adjusted for non-lipid risk factors and for lipoprotein concentrations.
These studies suggest that only a small percentage of individuals in a predominantly white North American population would be reclassified into a higher or lower ATP III risk category if the apoB/A-1 ratio were routinely substituted for or added to the total/HDL cholesterol ratio in clinical practice. It is unclear if similar results would be obtained in more diverse populations than these two studies. Also, whether there are particular subgroups of individuals, such as those with cardiometabolic risk factors (e.g. diabetes, hypertriglyceridemia) or familial clustering of disease, in whom CVD risk assessment and treatment could be improved using measures from advanced lipoprotein testing has not been well defined and should be investigated further.9 A clear need also remains for performing cost-benefit analyses that evaluate a 1- or 2-step process of obtaining a standard lipid profile along with apoB (or NMR LDL particle concentration).
1.B. LDL Particle Size: Are small LDL particles inherently more atherogenic than large particles?
Advocates of advanced lipoprotein testing may point out that small LDL particles (sometimes referred to as “dense” or Pattern B) are more atherogenic that large LDL particles (also referred to as “buoyant” or Pattern A); hence, identifying individuals with a greater concentration of small LDL particles may improve CVD risk assessment or therapeutic decisions. This section examines the evidence for the relative atherogenicity of LDL particles. Data to support the alternative view, namely that both small and large LDL particles are similarly atherogenic, will also be presented.
First, pathophysiological data supporting the view that small LDL particles may be more atherogenic than large particles include the greater oxidation potential of small particles, their association with endothelial dysfunction and multiple metabolic abnormalities, and impaired clearance from the circulation.1,41 However, several mechanisms may underlie the atherosclerotic effect of large LDL.1 At both extremes of LDL size, there is decreased receptor-binding affinity for LDL receptors.42 Large LDL also have higher core cholesterol ester content, potentially delivering more cholesterol per particle to arterial walls.43 Large LDL predominate in patients with familial hypercholesterolemia and those consuming high saturated fat diets.44
Second, epidemiological data for small LDL particles being more atherogenic than large ones is derived from studies that found that small LDL size was associated with higher CVD risk, although not all studies found this association to be independent of other risk factors such as triglycerides or apoB, with which small LDL particles are strongly associated.45–47 Other studies found that large LDL size was associated with CVD.48,49 An important limitation of these studies is that LDL size was often measured using GGE, which determines the predominant distribution of LDL size (i.e. average LDL size; large versus small) but does not quantify the number of large and small LDL particles.
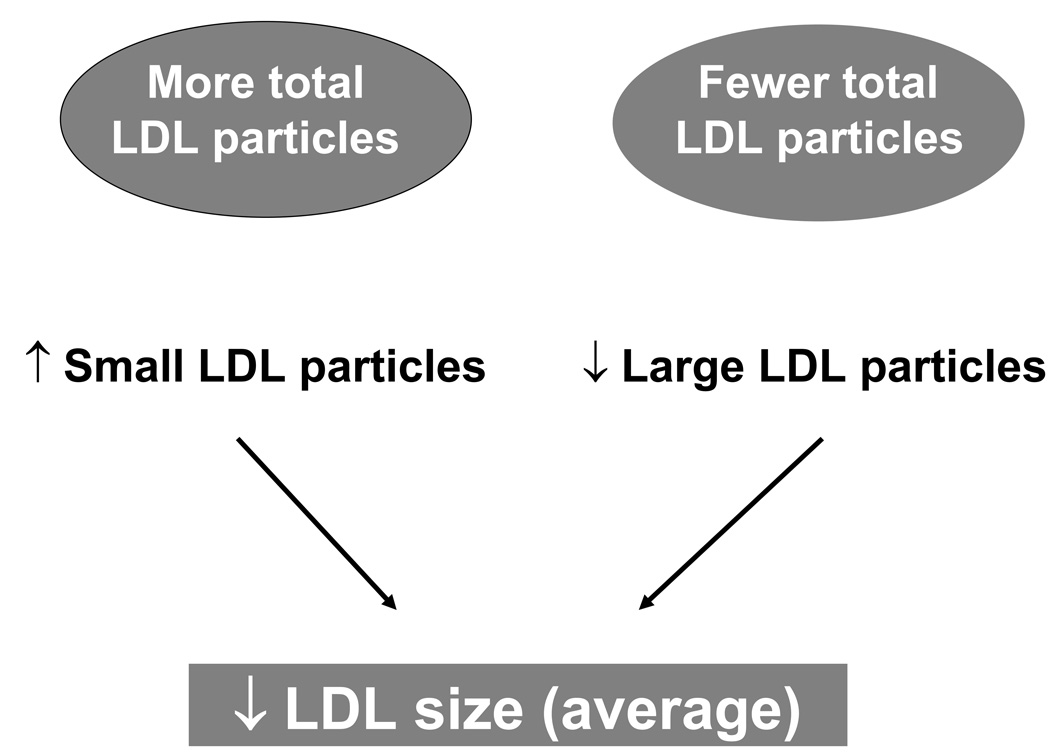
This distinction is important because a decrease in average LDL size does not necessarily translate into a greater number of small LDL particles, since it could also result from having fewer large LDL particles (Figure 3). Thus, small LDL size may potentially confound the association of LDL particle concentration (number) with CVD. A confounding variable is both associated with the risk factor and causally associated with the outcome. A potential confounder (e.g. smaller LDL size) may mask the relationship between the risk factor (e.g. LDL particle concentration) and the outcome (e.g. CVD). A study population may have a mix of individuals, some with predominantly large LDL such as those with familial hypercholesterolemia, and others with predominantly small LDL, such as those with diabetes or insulin resistance. These individuals would also be expected to differ in their concentration of LDL particles. For example, we may not know if risk in the diabetic is due to the diabetes or the small LDL particles, but if the risk is predicted correctly by knowing that the person has diabetes, high LDL particle concentration, high triglycerides, and low HDL cholesterol, it may not be useful to also measure LDL size.
Figure 3.
Smaller LDL size can result from fewer large LDL particles or more small LDL particles, showing the close relationship of LDL size with LDL particle concentration.
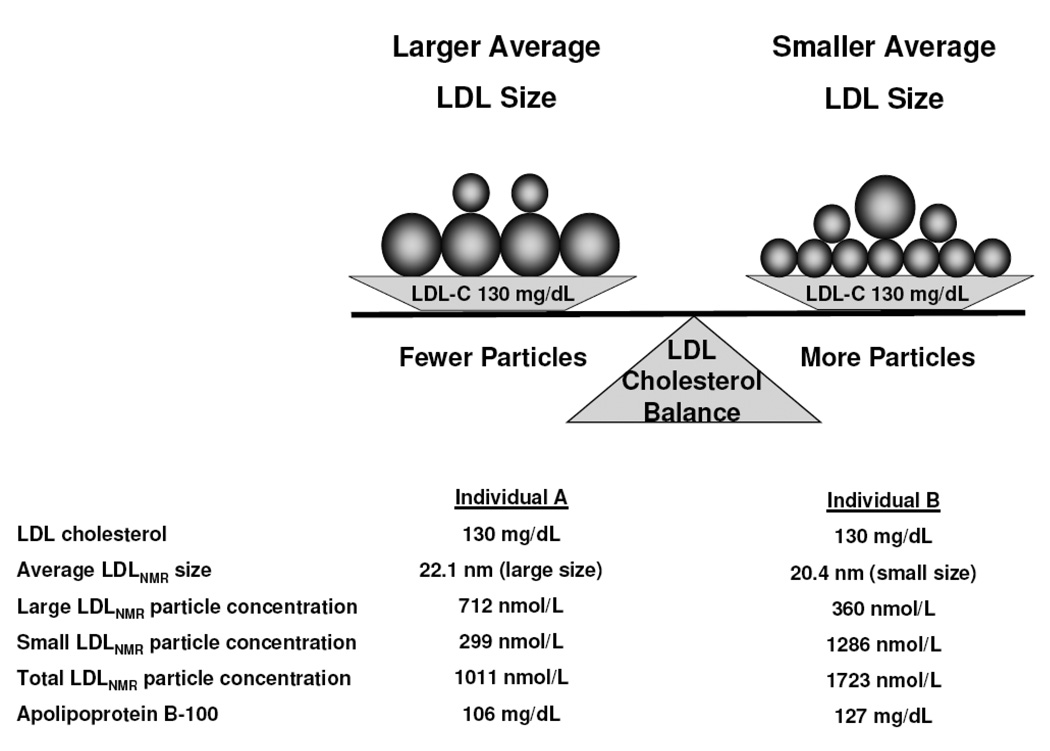
This is demonstrated by two case examples outlined in Figure 4. For the same serum concentration of LDL cholesterol, individual B has smaller average LDL particle size and concomitantly more LDL particles than individual A who has larger average LDL size. Small LDL particles contain less cholesterol than large ones. Thus, individual B must have a higher concentration of total LDL particles as measured either by NMR (LDL particle concentration) or immunoassay (apoB), for the same LDL cholesterol as individual A. Prior studies that suggested smaller LDL particles were more “atherogenic” did not adequately control for the inverse correlation between small and large LDL particle concentrations and potential confounding due to their differing associations with other lipoproteins, lipids, and cardiovascular risk factors.16,20,50
Figure 4.
Two individuals may have an identical LDL cholesterol level, but differ with respect to other measures of LDL, such as size and particle concentration.
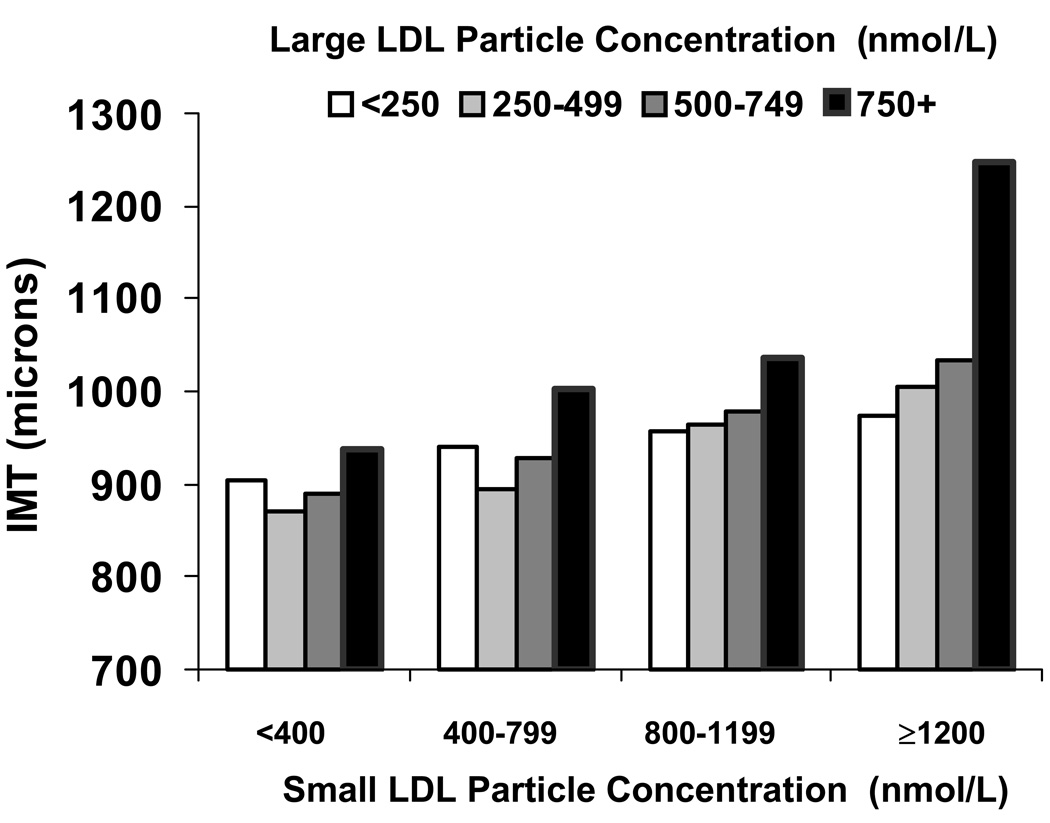
A report from ~5,500 asymptomatic individuals in the Multi-Ethnic Study of Atherosclerosis (MESA) compared the per-particle associations of small and large LDL with carotid intima-media thickness (IMT), a direct and well-validated measure of subclinical atherosclerosis.28 In order to unmask the association of large LDL with IMT, participants were classified into categories of small LDL particle concentration (Figure 5). In these stratified analyses, higher concentrations of large LDL were significantly associated with IMT within any particular category of small LDL. This was also confirmed by regression analysis: after accounting for particle correlations, both small and large LDL were “atherogenic” to a similar extent.
Figure 5.
Higher concentration of either large or small LDL were both positively associated with carotid intima-media thickness (IMT; Y-axis) in a multi-ethnic study of asymptomatic individuals (N=5,538), MESA Study.28
This was subsequently confirmed in relation to clinical CVD events in both primary prevention (Women’s Health Study; Figure 2: adjusted hazard ratios of 1.44 and 1.63 for large and small LDL, respectively, quintile 5 vs 1)34 and in secondary prevention (VA-HIT study).51 After accounting for lipoprotein correlations, particularly the inverse correlation between large and small LDL, both LDL subclasses showed similar associations with CVD events.34,51 LDL size was no longer associated with CVD after adjusting for LDL particle concentration and other risk factors (Figure 2: adjusted hazard ratio of 1.56 for smallest quintile of LDL size became attenuated to 1.06 after adjusting for LDL particle concentration, and was no longer significant). These findings are also consistent with a prior analysis from the Quebec Cardiovascular Study in which average LDL size, measured by GGE, did not add risk information after accounting for apoB (the latter reflecting the number of LDL particles).52
Future studies of LDL size should account for correlations of LDL size with standard lipids (especially triglycerides and HDL cholesterol) and, importantly, for the correlation of LDL size with LDL particle concentration or apoB.28 Therefore, smaller LDL size appears to be positively associated with CVD, not necessarily because small LDL are inherently more atherogenic than large ones, but probably because individuals with predominantly small LDL size also have more LDL particles. But if LDL size is a surrogate for LDL particle concentration, then why not use instead non-HDL cholesterol, which is also a surrogate for LDL particle concentration and can be obtained without additional cost?
2. Is advanced lipoprotein testing useful for detecting or managing “residual risk”?
The relative risk reduction in cardiovascular events with statin therapy is ~30%.4 Thus, approximately 70% of the patients who otherwise would have had CVD events still experience CVD events on statin therapy. Whether or not advanced lipoprotein tests may aid in detecting and treating this “residual risk” is uncertain.
Why do patients treated with statins have CVD events? First, risk factors for CVD often cluster, such that individuals with dyslipidemia may also have a number of cardiovascular risk factors, such obesity, hypertension, and diabetes. Even when low LDL cholesterol levels are achieved, other risk factors may be present that increase CVD risk. Also, treated patients remain at risk because they have higher levels of atherosclerosis, since their lipids may have been elevated for a long time prior to initiation of therapy. While statins lower LDL and total cholesterol by ~20–50%, they have a smaller effect on lowering triglycerides (~10–30%) and raising HDL cholesterol (~5–10%). Numerous studies have shown that low HDL cholesterol and high triglycerides are risk factors independent of LDL cholesterol.4
A secondary analysis from PROVE-IT TIMI 22 found that on-treatment levels of triglycerides were independently associated with recurrent coronary events, with triglycerides <150 mg/dL associated with 20% relative risk reduction after adjusting for risk factors.53 This may be particularly relevant in patients with diabetes or metabolic syndrome in whom elevated triglycerides are common.
The ADA/ACC consensus statement advocated the use of non-HDL cholesterol and apoB (or NMR LDL particle concentration) in addition to LDL cholesterol in subjects at increased metabolic risk, although the targets for apoB were somewhat arbitrary (LDL cholesterol <70 mg/dL, non-HDL cholesterol <100 mg/dL, apoB <80 mg/dL, for those at highest risk).9 A recent study suggested that in statin-treated patients, lower targets of LDL and non-HDL cholesterol may be needed to achieve the same target apoB level compared with untreated patients.54 Lowering of therapeutic targets for LDL cholesterol and non-HDL cholesterol may diminish the residual risk on therapy. More data is clearly needed on the utility of advanced lipoprotein tests as well as non-HDL cholesterol, total/HDL cholesterol ratio, or triglycerides, as targets of therapy together with LDL cholesterol lowering.
Summary of current limitations to the clinical utility of advanced lipoprotein tests
In summary, a lipoprotein measure obtained from an advanced test is clinically useful if the following criteria are met: it adds to clinical knowledge; it provides risk information that is independent of established predictors; it is easy to measure and interpret in a clinical setting; it is accurate, reproducible and internationally standardized; and it has a favorable cost–benefit ratio.55 Importantly, the lipoprotein measures should improve patient management, particularly through more accurate risk classification and guiding choice of therapy.55 Challenges to the routine clinical use of advanced lipoprotein tests are summarized in the Table. To date, the most useful measures obtained from lipoprotein tests relate to atherogenic particle number (apoB or LDL particle concentration), but for now, non-HDL cholesterol or the total/HDL cholesterol ratio are of comparable predictive strength and result in no additional cost. LDL size is correlated with atherogenic particle number, but it has no clear benefit over measuring particle number in most studies to date. Additional research is needed to determine the utility of following changes in lipoproteins as therapeutic targets and whether certain subgroups of individuals, such as those with cardiometabolic risk or familial syndromes, may particularly benefit. While advanced lipoprotein testing and subfractionation may not be ready yet for routine clinical use, the methods are important for advancing research, developing potentially novel therapies, and understanding the pathophysiology of atherothrombotic diseases.
Table 1.
Summary of Current Limitations to the Clinical Utility of Advanced Lipoprotein Tests
| Lack of standardization and comparability of information provided by various tests |
| Information overload can be minimized by focusing on several key lipoprotein measures |
| Accessibility |
| Demonstration that tests alter clinical management of patients, such as improving risk classification or targeting of therapy |
| Identifying subgroups of individuals who may particularly benefit from testing (e.g. those with cardiometabolic risk factors) |
| Evaluation of cost-benefit ratio |
Supplementary Material
Footnotes
Disclosures
Dr. Mora has received research grant support from the American Heart Association (0670007N), Sandra A. Daugherty Foundation, National Heart, Lung, and Blood Institute (K08 HL094375), Lerner Research Young Investigator Award, Merck and AstraZeneca, and speaking honorarium from Pfizer for an educational (non-promotional) activity.
Mora. Advanced lipoprotein testing and subfractionation
References
- 1.Sacks FM, Campos H. Clinical review 163: Cardiovascular endocrinology: Low-density lipoprotein size and cardiovascular disease: a reappraisal. J Clin Endocrinol Metab. 2003;88:4525–4532. doi: 10.1210/jc.2003-030636. [DOI] [PubMed] [Google Scholar]
- 2.Mudd JO, Borlaug BA, Johnston PV, Kral BG, Rouf R, Blumenthal RS, Kwiterovich PO., Jr Beyond low-density lipoprotein cholesterol: defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol. 2007;50:1735–1741. doi: 10.1016/j.jacc.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 3.Blaha MJ, Blumenthal RS, Brinton EA, Jacobson TA. The importance of non-HDL cholesterol reporting in lipid management. Journal of Clinical Lipidology. 2008;2:267–273. doi: 10.1016/j.jacl.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed]
- 5.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF, Jr, Smith SC, Jr, Stone NJ, Taubert KA. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 6.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Cats VM, Orth-Gomer K, Perk J, Pyorala K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145–155. doi: 10.1016/j.atherosclerosis.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 7.McPherson R, Frohlich J, Fodor G, Genest J, Canadian Cardiovascular S. Canadian Cardiovascular Society position statement--recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can J Cardiol. 2006;22:913–927. doi: 10.1016/s0828-282x(06)70310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barter PJ, Ballantyne CM, Carmena R, Castro Cabezas M, Chapman MJ, Couture P, de Graaf J, Durrington PN, Faergeman O, Frohlich J, Furberg CD, Gagne C, Haffner SM, Humphries SE, Jungner I, Krauss RM, Kwiterovich P, Marcovina S, Packard CJ, Pearson TA, Reddy KS, Rosenson R, Sarrafzadegan N, Sniderman AD, Stalenhoef AF, Stein E, Talmud PJ, Tonkin AM, Walldius G, Williams KM. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247–258. doi: 10.1111/j.1365-2796.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 9.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51:1512–1524. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Rifai N, Warnick GR, Dominiczak MH. Handbook of Lipoprotein Testing. 2nd ed. Washington, DC: AACC Press; 2000. [Google Scholar]
- 11.NACB Laboratory Medicine Practice Guidelines. Emerging biomarkers of cardiovascular disease and stroke. [accessed Nov 28, 2008]; Draft version 91906. http://www.aacc.org/SiteCollectionDocuments/NACB/LMPG/Emerging_Risk_Factors/NACB_full_guidelines_draft_091906.pdf.
- 12.Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy--a review of the evidence. J Intern Med. 2006;259:493–519. doi: 10.1111/j.1365-2796.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 13.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23:97–104. [PubMed] [Google Scholar]
- 14.Kulkarni KR, Garber DW, Marcovina SM, Segrest JP. Quantification of cholesterol in all lipoprotein classes by the VAP-II method. J Lipid Res. 1994;35:159–168. [PubMed] [Google Scholar]
- 15.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48:171–180. [PubMed] [Google Scholar]
- 16.Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22:1175–1180. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 17.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am J Cardiol. 2002;90:22i–29i. doi: 10.1016/s0002-9149(02)02632-2. [DOI] [PubMed] [Google Scholar]
- 19.Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, Reitz RE, Krauss RM. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54:1307–1316. doi: 10.1373/clinchem.2007.100586. [DOI] [PubMed] [Google Scholar]
- 20.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 21.Witte DR, Taskinen MR, Perttunen-Nio H, Van Tol A, Livingstone S, Colhoun HM. Study of agreement between LDL size as measured by nuclear magnetic resonance and gradient gel electrophoresis. J Lipid Res. 2004;45:1069–1076. doi: 10.1194/jlr.M300395-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Ensign W, Hill N, Heward CB. Disparate LDL phenotypic classification among 4 different methods assessing LDL particle characteristics. Clin Chem. 2006;52:1722–1727. doi: 10.1373/clinchem.2005.059949. [DOI] [PubMed] [Google Scholar]
- 23.Stein EA. Are measurements of LDL particles ready for prime time? Clin Chem. 2006;52:1643–1644. doi: 10.1373/clinchem.2006.073452. [DOI] [PubMed] [Google Scholar]
- 24.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298:786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 25.Thompson A, Danesh J. Associations between apolipoprotein B, apolipoprotein AI, the apolipoprotein B/AI ratio and coronary heart disease: a literature-based meta-analysis of prospective studies. J Intern Med. 2006;259:481–492. doi: 10.1111/j.1365-2796.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 26.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 27.Sniderman AD, Furberg CD, Keech A, Roeters van Lennep JE, Frohlich J, Jungner I, Walldius G. Apolipoproteins versus lipids as indices of coronary risk and as targets for statin treatment. Lancet. 2003;361:777–780. doi: 10.1016/s0140-6736(03)12663-3. [DOI] [PubMed] [Google Scholar]
- 28.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr, O'Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJ, Khaw KT, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 30.Cromwell WC, Otvos J, Keyes MJ, Pencina MJ, Sullivan D, Vasan RS, Wilson PWF, D'Agostino RB. LDL particle number and risk for future cardiovascular disease in the Framingham Offspring Study - implications for LDL management. J Clin Lipidol. 2007;1:583–592. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walldius G, Jungner I, Aastveit AH, Holme I, Furberg CD, Sniderman AD. The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin Chem Lab Med. 2004;42:1355–1363. doi: 10.1515/CCLM.2004.254. [DOI] [PubMed] [Google Scholar]
- 32.Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112:3375–3383. doi: 10.1161/CIRCULATIONAHA.104.532499. [DOI] [PubMed] [Google Scholar]
- 33.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, Pencina MJ, Schoonmaker C, Wilson PW, D'Agostino RB, Vasan RS. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–785. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 34.Mora S, Otvos J, Rifai N, Rosenson R, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119 doi: 10.1161/CIRCULATIONAHA.108.816181. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller M, Ginsberg HN, Schaefer EJ. Relative atherogenicity and predictive value of non-high-density lipoprotein cholesterol for coronary heart disease. Am J Cardiol. 2008;101:1003–1008. doi: 10.1016/j.amjcard.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 36.Sniderman AD. We must prevent disease, not predict events. J Am Coll Cardiol. 2008;52:300–301. doi: 10.1016/j.jacc.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 38.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, Yusuf S. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 40.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 41.Tribble DL, Rizzo M, Chait A, Lewis DM, Blanche PJ, Krauss RM. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. Am J Med. 2001;110:103–110. doi: 10.1016/s0002-9343(00)00700-2. [DOI] [PubMed] [Google Scholar]
- 42.Campos H, Arnold KS, Balestra ME, Innerarity TL, Krauss RM. Differences in receptor binding of LDL subfractions. Arterioscler Thromb Vasc Biol. 1996;16:794–801. doi: 10.1161/01.atv.16.6.794. [DOI] [PubMed] [Google Scholar]
- 43.Rudel LL, Parks JS, Hedrick CC, Thomas M, Williford K. Lipoprotein and cholesterol metabolism in diet-induced coronary artery atherosclerosis in primates. Role of cholesterol and fatty acids. Prog Lipid Res. 1998;37:353–370. doi: 10.1016/s0163-7827(98)00015-0. [DOI] [PubMed] [Google Scholar]
- 44.Patsch W, Ostlund R, Kuisk I, Levy R, Schonfeld G. Characterization of lipoprotein in a kindred with familial hypercholesterolemia. J Lipid Res. 1982;23:1196–1205. [PubMed] [Google Scholar]
- 45.Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–888. [PubMed] [Google Scholar]
- 46.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–881. [PubMed] [Google Scholar]
- 47.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95:69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- 48.Hulthe J, Wiklund O, Bondjers G, Wikstrand J. LDL particle size in relation to intima-media thickness and plaque occurrence in the carotid and femoral arteries in patients with hypercholesterolaemia. J Intern Med. 2000;248:42–52. doi: 10.1046/j.1365-2796.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 49.Campos H, Moye LA, Glasser SP, Stampfer MJ, Sacks FM. Low-density lipoprotein size, pravastatin treatment, and coronary events. JAMA. 2001;286:1468–1474. doi: 10.1001/jama.286.12.1468. [DOI] [PubMed] [Google Scholar]
- 50.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2002;90:89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 51.Otvos J, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. LDL and HDL particle subclasses predict coronary events and are changed favorable by gemfibrozil therapy in the Veterans Affairs HDL Intervention Trial (VA-HIT) Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 52.Lamarche B, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP, Prospective results from the Quebec Cardiovascular Study Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Arterioscler Thromb Vasc Biol. 1997;17:1098–1105. doi: 10.1161/01.atv.17.6.1098. [DOI] [PubMed] [Google Scholar]
- 53.Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–730. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 54.Ballantyne CM, Raichlen JS, Cain VA. Statin therapy alters the relationship between apolipoprotein B and low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol targets in high-risk patients: the MERCURY II (Measuring Effective Reductions in Cholesterol Using Rosuvastatin) trial. J Am Coll Cardiol. 2008;52:626–632. doi: 10.1016/j.jacc.2008.04.052. [DOI] [PubMed] [Google Scholar]
- 55.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.