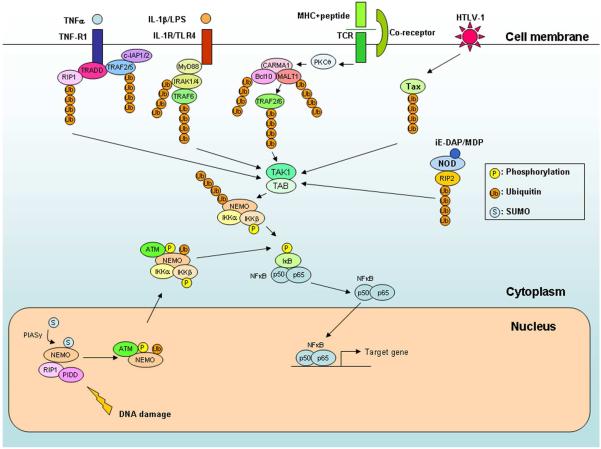
Figure 2. Expanding role of ubiquitination in the activation of TAK1 and IKK by diverse NF-κB signaling pathways.
NF-κB is activated by many different signaling pathways that converge on TAK1 and IKK complexes. These pathways include those emanating from cell surface receptors, including TNF receptor (TNF-R1), IL-1 receptor and Toll-like receptors (IL-1R/TLR), and T cell receptors (TCR), as well as those from intracellular receptors, such as NOD1 and NOD2. In addition, viral proteins such as Tax of human T cell leukemia virus-1 (HTLV-1) activate TAK1 and IKK in the cytosol. All of these pathways employ one or more TRAF proteins as the ubiquitin ligase(s) to catalyze K63 polyubiquitination of various signaling proteins, which activate the TAK1 kinase complex, leading to the activation of IKK and NF-κB. DNA damage in the nucleus can also activate IKK in the cytosol through a mechanism involving sequential sumoylation, phosphorylation and ubiquitination of NEMO (see text for details).