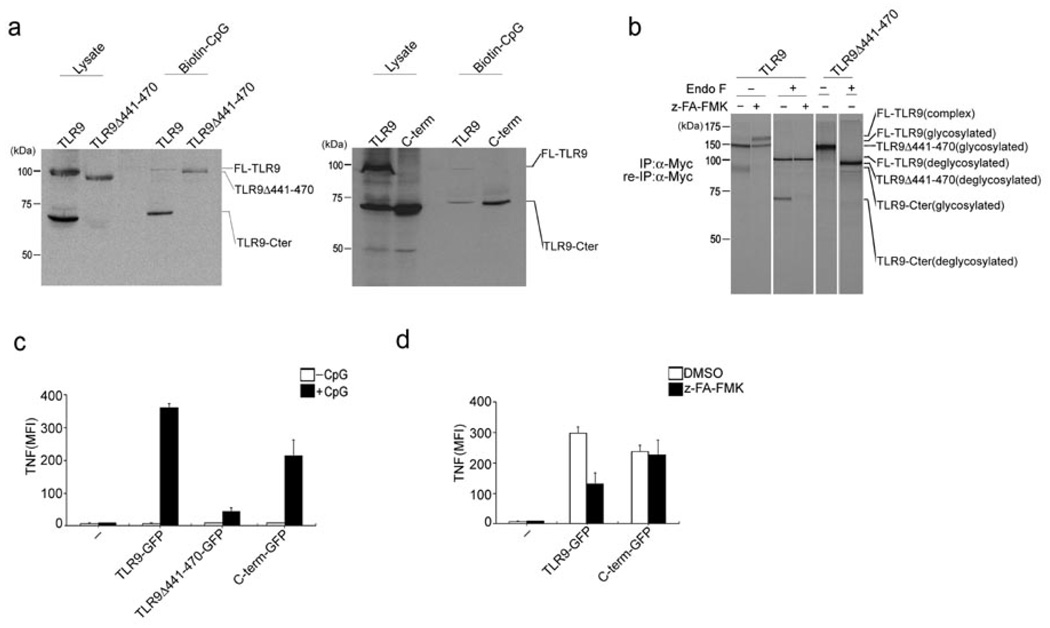
Figure 4.
The C-terminal TLR9 fragment is the active form responsible for binding CpG DNA and subsequent TLR9 signal transduction. (a) RAW macrophages stably expressing TLR9Δ441–470 or the C-terminal TLR9 fragment (471–1032) were incubated with 3 µM biotinylated CpG DNA for 3 h at 37°C. CpG DNA and materials bound to it were precipitated with streptavidin agarose, subjected to digestion with EndoF and precipitates and input lysates were immunoblotted with anti-Myc. (b) Myc-tagged wild-type TLR9 and Myc-tagged TLR9Δ441–470 were immunoprecipitated and reimmunoprecipitated with anti-Myc from DMSO- or z-FA-FMK-treated and metabolically labeled RAW macrophages, digested with EndoF where indicated, and visualized by SDS-PAGE. (c,d) BMDCs from Tlr9−/− mice were retrovirally transduced with vectors encoding GFP-tagged wild-type TLR9, TLR9Δ441–470 or the C-terminal TLR9 fragment (471–1032). (c) Cells were stimulated with CpG DNA (1 µM) for 4 h in the presence of brefeldin A at day 5 of BMDC culture. Cells were fixed and stained with anti-TNF, and TNF-expressing GFP+ cells were quantified by flow cytometry. Data were generated from three independent experiments and are expressed as mean fluorescence intensity (MFI). (d) Cells were stimulated with CpG (1 µM) for 4 h in the presence of DMSO or 1 µM z-FA-FMK for 8 h at day 5 of BMDC culture. TNF expression was measured as in (c). Data are representative of three independent experiments (c,d; average, s.d.).