Abstract
Transungual delivery of antifungal drugs is hindered by the low permeability of human nail plates, and as such, repeated dosing over a long period of time is necessary for effective treatment. The objectives of this study were to explore the possibilities of (a) enhancing the delivery of ciclopirox (CIC) across human nail plates and (b) sustaining CIC delivery from the larger resultant drug depot in the nail plates with constant voltage iontophoresis. In vitro passive and 9 V cathodal iontophoretic transport experiments of CIC across human nails were performed. Transungual CIC delivery with Penlac® was the control. The amounts of CIC released from and deposited in the nails were determined in drug release and extraction experiments, respectively. Iontophoresis increased the flux of CIC permeated across the nail approximately 10 times compared to passive delivery from the same formulation or from Penlac®. A significant amount of CIC was loaded into and released from the nails; the CIC concentrations were estimated to be above the minimum inhibitory concentrations of CIC for dermatophytic molds. The apparent transport lag time decreased in iontophoretic transport. The results demonstrate that iontophoresis was able to deliver an effective amount of CIC into and across the nails, and this suggests the feasibility of a constant voltage battery-powered transungual iontophoretic device.
Keywords: iontophoresis, ciclopirox, human nail plate, constant voltage, transungual delivery
INTRODUCTION
Onychomycosis is the most common nail disease affecting 2-13% of the general population.1 Dermatophytic molds such as Trichophyton rubrum and Trichophyton mentagrophytes account for approximately 85% of onychomycosis cases, nondermatophytic molds account for 15% of onychomycosis cases, and yeasts rarely cause onychomycosis.2 Infections range from super-ficial, causing discoloration of the nail plate, to severe, resulting in the loss of the nail plate together with deformities of the affected areas. Nail fungal infections are more than a cosmetic problem. They cause physical discomfort and are associated with social and emotional consequences.3 According to the American Academy of Dermatology (www.aad.org), onychomycosis has a substantial negative impact on the patients' quality of life. Furthermore, untreated nails are susceptible to secondary infections, particularly in patients with impeded immune systems.
Onychomycosis is frequently treated with the oral administration of antifungal drugs such as griseofulvin (Grisactin®), ketoconazole (Nizarol®), itraconazole (Sporanox®), terbinafine (Lamisil®), and off-label fluconazole (Diflucan®). As these medications are ingested for 6 weeks or more, systemic toxicity is a concern and periodic checking of patients' liver biochemistry is recommended.4 Topical therapies eliminate such adverse effects and improve patient compliance. Unfortunately, topical treatment can only be successfully employed for mild infections and occasionally for acute onychomycosis due to the barrier properties of the nail. Newer nail lacquer formulations such as ciclopirox (Penlac®) and amorolfine (Curanail®) are more effective than traditional topical dosage forms. Penlac® is the only topical product in the U.S. market indicated for the treatment of onychomycosis. The delivery system promotes nail penetration by providing a high concentration gradient across the nail after evaporation of volatile solvents in the lacquer.5 Still, the mycological cure rate was reported to be less than 50%.6 Relapse of the diseases or re-infection is also common.7,8
The efficacy of topical therapy is greatly limited by the low permeability of the nail plate. Pharmaceutical scientists have been trying different chemical methods to enhance transungual delivery. The physicochemical properties of antifungal drugs were studied to screen for drugs suitable for topical therapy.9,10 Potent oxaborole antifungal drugs were tested for transungual penetration in vitro.11 A number of chemical enhancers have been investigated to facilitate transungual drug penetration.12-14 Novel formulations were developed for the topical treatment of onychomycosis.15,16 In spite of these efforts, sufficient amounts of drug are still not deliverable into and across the nail. Physical enhancement methods such as iontophoresis may overcome the drawbacks associated with the existing approaches and allow the drug to be delivered across the nail to attain the drug minimum inhibitory concentrations. Moreover, iontophoresis may possess bacteriostatic and fungistatic properties.17
Iontophoresis is a method to enhance the delivery of compounds across a membrane by means of an electric field. The mechanisms of iontophoresis-enhanced transport include electrophoresis (direct field effect or Nernst-Planck effect),18 electroosmosis (convective solvent flow),19 and electropermeabilization (field-induced membrane alteration and an increase in membrane permeability).20,21 Several iontophoretic products have been marketed for transdermal and topical drug delivery, namely Phoresor® (Iomed Inc., Salt Lake City, UT), Actyve™ (Vyteris Inc., Fair Lawn, NJ), and IONSYS E-TRANS® (Alza Corp., Mountain View, CA). The wearable electronic disposable delivery (WEDD) system from Birch Point Medical (now Travanti Pharma, Mendota Heights, MN) is a thin, band-aide size low-current iontophoresis system.
Despite the potential benefits of using iontophoresis in the treatment of onychomycosis, there are few studies on transungual iontophoresis.22-24 In our previous study,25 electrophoresis was shown to be the dominant driving force in the transungual iontophoretic transport of small permeants across fully hydrated nail plates. Contribution of electroosmosis to transungual electrotransport was less than 10% of that from electrophoresis for small permeants at pH 7.4 and ionic strength of 0.16 M, and such contribution remained small when the conditions varied from pH 3 to 9 and ionic strength from 0.04 to 0.7 M.26 The size exclusion effect of the nail plate was important in determining the permeability of the nail.27 No significant structure alteration of the nail was observed under the studied electric current conditions of 0.1 and 0.3 mA across 0.64 cm2 nail surface. A constant voltage iontophoretic system with a relatively small battery, which has some practical advantages over a constant current iontophoresis system, can therefore be used in transungual antifungal drug delivery. These advantages include a smaller device design similar to the WEDD system and the reduction in manufacturing cost for a small disposable transungual patch.
The objective of the present study was to explore the feasibility of a portable battery-driven iontophoretic system to deliver a drug into and across the human nail plate. Ciclopirox (CIC), molecular weight (MW) of 207.3 Da and clog P of 2.0,11 was selected as the model antifungal drug. Cathodal transungual iontophoretic transport of CIC from an aqueous ethanol solution was carried out using a 9 V alkaline portable battery as the power supply. Passive transport using the same ethanol formulation and that using Penlac® were the controls. The release of CIC from the nail plate after passive and iontophoretic delivery was investigated, and the amount of CIC left in the nail plate was determined after the release experiment. The feasibility of enhancing the delivery of CIC across human nail plates and sustaining CIC delivery from the resultant drug depot in the nail plates with constant voltage iontophoresis was examined.
EXPERIMENTAL
Materials
Ciclopirox olamine (MW 268.4 Da, approximately 97% purity) was purchased from Sigma-Aldrich (St. Louis, MO). Penlac® nail lacquer was purchased from Sanofi Aventis (Bridgewater, NJ). Phosphate-buffered saline (PBS) of pH 7.4 (0.01 M phosphate buffer, 0.0027 M potassium chloride, and 0.137 M sodium chloride) was prepared by dissolving PBS tablets (Sigma-Aldrich) in distilled, deionized water. Sodium azide (99% purity, Acros, Morris Plains, NJ) of 0.02% (w/v) was added to PBS as a bacteriostatic agent. Bovine serum albumin (BSA, fraction V, Fisher Scientific, Fair Lawn, NJ) of 5% (w/v) was prepared by dissolving BSA in PBS. Ethanol (denatured, anhydrous ethyl alcohol) was purchased from Fisher Scientific (Rochester, NY). All materials were used as received.
Nail Sample Preparation
Human cadaver fingernail plates (from nine donors, male and female, age 24-90) were obtained from Science Care Anatomical (Phoenix, AZ). Nails from all fingers were used but were excluded when they were too small to fit in the diffusion cell setup described in “CIC Transport Experiments” below. Up to 10 nail plates were used in each experiment. The frozen nail plates were thawed at room temperature. Adhering tissues on the nail plates were removed with a pair of forceps. The nail plates were then rinsed with distilled, deionized water. The clean nail plates were immediately used in the transport experiments without preequilibration to full hydration in PBS. This would better mimic the partially hydrated nail condition normally encountered in vivo. The thickness of the nail plates, ranging from 0.4 to 1.0 mm, was measured using a micrometer (Mitutoyo, Kawasaki, Kanagawa, Japan) at the end of the experiments. The use of human tissues was approved by the Institutional Review Board at the University of Cincinnati, Cincinnati, OH.
Strategy
A side-by-side diffusion cell setup was employed in the present study to examine transungual iontophoresis under occlusion. In a previous study, nail water uptake and evaporation were observed to be fast: the nails approached 90% of complete hydration in half an hour after the nails were soaked in water.25 The effective diffusion coefficient of water in the nails could be up to 3×10-7 cm2/s at 100% relative humidity28 and transonychial water loss was reported to be from 19 to 75 g/m2/h in healthy subjects.29-31 The present experimental setup mimicked the condition when the nails were occluded in practice—the ventral side of the nail was in contact with an aqueous environment while the dorsal side of the nail was in contact with the ethanol/water drug system. The nails were maintained under this condition over the duration of the delivery study similar to that in an iontophoresis patch application.
Passive and iontophoretic transport of CIC were performed for 24 h to determine the amount of CIC permeated across the nails with the same ethanol/water formulation. The same duration of 24 h allowed a direct comparison of the results in the iontophoretic and passive transport experiments as the drug dosing duration could affect drug loading into the nail plate. Passive transport experiments were also carried out for 94 h to determine the steady-state flux and transport lag time. To examine the release of CIC from the nails postadministration, release experiments were conducted immediately after passive and iontophoretic delivery and the removal of the tested solution from the nails. The Penlac® lacquer experiments were divided into two stages. In the first stage, the formulation was applied and the permeation of CIC was monitored over 24 h for direct comparison with the CIC data obtained in the above passive and iontophoretic studies. The second stage mimicked the above release experiments but without the removal of the lacquer from the nail surface. At the end of the release experiments, the amounts of CIC in the nails were determined in the extraction study. The CIC concentrations in the nail plates were estimated and compared with the minimum inhibitory concentrations of CIC.
CIC Transport Experiments
The nail plates were mounted between side-by-side diffusion cells (HazalGlas, Cincinnati, OH) with custom-made nail adapters to fit the curvature of the nail plates. The dorsal side of the nail plates faced the donor chamber and the ventral side of the nail plates faced the receptor chamber. The diffusion cells had an effective diffusion area of approximately 0.64 cm2 and a cell volume of 2 mL. The adapters, which had a similar circular opening of 0.64 cm2 in the center, were constructed from silicone elastomer (MED-6033, NuSil Silicone Technology, Carpinteria, CA). CIC is slightly soluble in water and has a pKa of 7.2. The donor solution was 12.5 mg/mL (approximately 0.05 M) CIC olamine in ethanol/water (30:70, v/v) and had pH of 8.7. Ethanol was added into the donor solution to increase the solubility of CIC. The receptor solution was 5% (w/v) BSA in PBS 32 to mimic the condition of an extracellular fluid. Preliminary studies also showed that the addition of BSA effectively prevented CIC from binding to the nail adapter.
In iontophoretic transport experiments, a constant voltage of 9 V was applied across the nail plates directly with an alkaline battery. Pt electrode was used in the donor as the cathode, and Ag electrode was the anode in the receptor (both connected to wiring attached to the battery). Ag/AgCl was not used as the cathode because CIC olamine in the donor was found to interact with Ag/AgCl, resulting in AgCl peeling from the electrode surface. The receptor pH was approximately 7.4 checked at the beginning and the end of the experiments. The donor pH increased to 10-11 at the end of the iontophoretic transport experiments due to the hydroxide ion generated at the Pt electrode. The fraction of ionized CIC in the donor chamber therefore increased from 96% in the beginning to close to 100% at the end of the iontophoresis experiments. The relatively high pH was also shown not to significantly affect the structure of the nail in terms of nail porosity (data not shown). The voltage drop across the nail plate was monitored using a multimeter (Fluke 73III, Everett, WA) and was found to be constant (≈9 V) over the course of the experiment. The electric current across the nail plate was measured near the end of the transport experiment by the voltage drop across a fixed resistor (147 Ω) connecting in series to the nail plate in the electric circuit using Ohm's law. With the assumption that the overpotential at the electrode was negligible, the electrical resistances of the nail plates were then estimated using the voltage drop, electric current, and Ohm's law. At predetermined time intervals, 1 μL of donor solution and 1 mL of receptor solution were taken for assay; 1 mL fresh receptor solution was added to maintain a constant volume in the receptor. Passive transport experiments of CIC were carried out under the same donor and receptor conditions. In the transport experiments with Penlac®, 30 μL of Penlac® lacquer (approximately 2.4 mg CIC) was applied to the dorsal surface of the nail plate and allowed to dry for 1 h. Fresh receptor solution was then added into the receptor chamber to start the transport experiments. The duration of the iontophoretic and Penlac® transport experiments was 24 h and the durations of the passive transport experiments were 24 and 94 h. Transport experiments were conducted at room temperature (20±2°C).
The cumulative amount of CIC transported across the nail plate (Q) was plotted against time (t); the steady-state flux of CIC (J) and apparent lag time (tL) were calculated from the slope and the x-intercept of the linear portion of the plot, respectively:
| (1) |
where A is the diffusion area and ΔQ/Δt is the slope of the cumulative amount against time. The enhancement factor (E) is expressed as the ratio of the iontophoretic flux over the passive flux.
CIC Release Experiments
At the end of the transport experiments, the donor and receptor solutions were removed and both chambers were rinsed with deionized water three times. In the Penlac® study, the lacquer was not removed from the nail dorsal surface and the donor chamber was not rinsed. Release experiments were then performed by adding fresh receptor solution of 2 mL to the receptor chamber while leaving the donor chamber empty. Aliquots of receptor solutions (1 mL) were taken for assay at predetermined time intervals and replaced with fresh receptor solution to maintain a constant volume in the receptor. The duration of the release experiments was 50 h.
CIC Extraction Experiments
At the end of the release experiments, the nail plates were rinsed with distilled, deionized water and blotted dry with Kimwipes®. For the Penlac®-treated nail plate, the lacquer was removed from the nail dorsal surface with ethanol. The nail plates were then soaked in 2 mL ethanol/PBS solution (30:70, v/v). After 24 h extraction, the nail plates were blotted dry with Kimwipes® and transferred into fresh 2 mL ethanol/PBS solution (30:70, v/v). The extraction solutions were assayed for the CIC amounts. The extraction procedure was repeated until the concentration of CIC in the extraction solution was less than 10% of that in the first extraction solution. The total amounts of CIC left in the nail plates were calculated. The recovery of this extraction method determined by equilibrating a known amount of CIC with nail plates in a preliminary study was 98±6% (n=3). This recovery result also implied no significant irreversible binding of CIC to the nail plate.
CIC and BSA Assay
The samples were spectrophotometrically assayed at 309 nm for CIC concentrations with a reference of the corresponding solutions but without CIC using a spectrophotometer (UV-mini 1240, Shimadzu, Kyoto, Japan). Calibration curves of CIC were constructed using solutions as those of the samples and were found to be linear from 2 to 20 μg/mL (r2 > 0.994). The receptor samples after 100-fold dilution were also assayed at 280 nm for BSA concentrations. The change in the BSA concentration in the receptor in the transport and release experiments was found to be generally less than 5%. CIC data would be excluded in the analysis when the change in BSA concentration significantly affected the CIC results and caused more than 40% uncertainty. In a preliminary study, the assay at 309 nm did not detect any noticeable amounts of substances from the nail plates (from the same human donors) in the 5% (w/v) BSA solution and ethanol/PBS solution (30:70, v/v) that would interfere with the assay of CIC over a 48-h equilibration period.
Statistical Analysis
The Mann-Whitney test was used to evaluate the significant differences among the different groups as the sample distributions were highly skewed due to the large sample-to-sample variability. Differences were considered to be significant at a level of p<0.05. The means±standard deviations (SD) of the data are presented.
RESULTS
CIC Permeation Across the Nail Plate
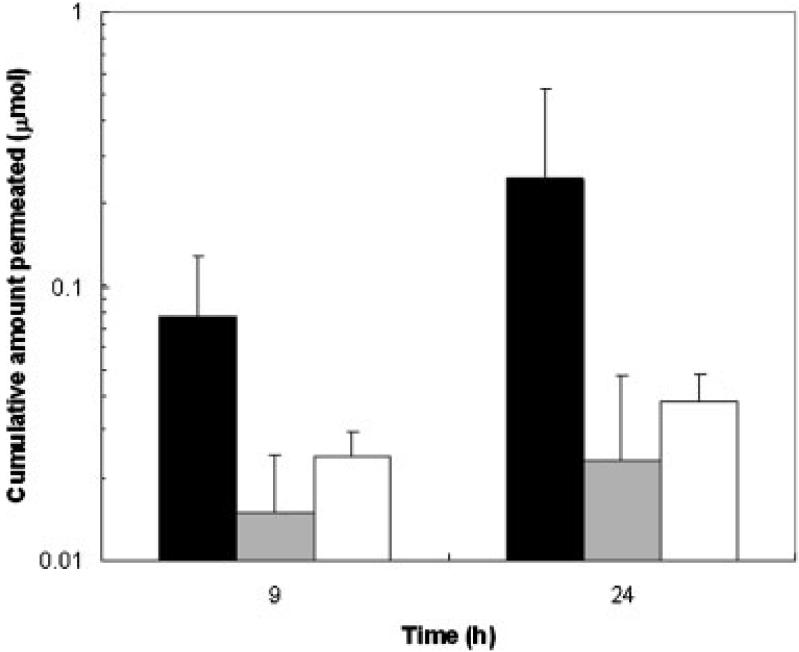
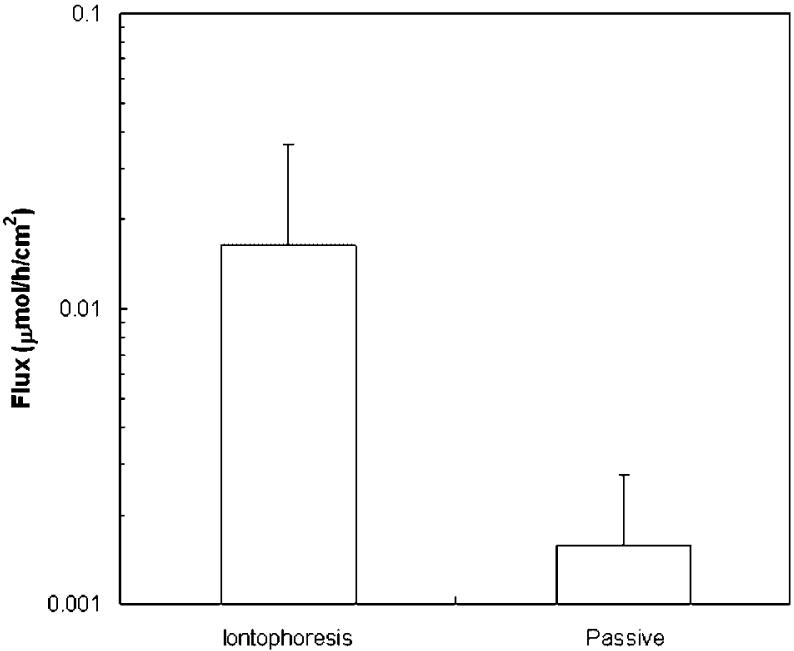
Figure 1 presents the cumulative amounts of CIC delivered across the nail plate at 9 h (Q9) and 24 h (Q24). The amounts of CIC delivered across the nail plates at 9 h were 0.08 μmol (0.02 mg) in the iontophoretic transport, 0.01 μmol (0.003 mg) in the passive transport, and 0.02 μmol (0.004 mg) in the Penlac® experiments. At 24 h, the amounts of CIC delivered across the nail plates increased to 0.2 μmol (0.05 mg) in the iontophoretic transport, 0.02 μmol (0.005 mg) in the passive transport, and 0.04 μmol (0.008 mg) in the Penlac® experiments. The data show that cathodal iontophoresis significantly increased the penetration of CIC across the nail compared to the Penlac® formulation (p<0.05). There was no significant difference between the passive transport of the tested formulation and Penlac® (p>0.05). The iontophoretic and passive fluxes of CIC were also determined (Fig. 2). The iontophoretic flux was 0.02 μmol/h/cm2 (3 μg/h/cm2) and was about 10 times higher than the passive flux (0.002 μmol/h/cm2 or 0.3 μg/h/cm2). Iontophoresis significantly enhanced the transport of CIC over passive delivery (p<0.05). The apparent lag time was also shortened from 20±7 h for passive transport to 2±2 h for iontophoretic transport (p<0.05). The electrical resistance of the nail plates was also determined near the end of the transport experiment. The average electrical resistance of the nail plate in the present study (34±23 kΩ) was approximately five times higher than that of the fully hydrated nail plates obtained in our previous studies (7±3 kΩ),25,27 suggesting that the nail plates were only partially hydrated as anticipated due to the presence of ethanol in the donor solution.
Figure 1.
Cumulative amounts of ciclopirox delivered across human nail plates at 9 h (Q9) and 24 h (Q24) in constant 9 V cathodal iontophoretic (dark bars), passive (gray bars), and Penlac® (open bars) transport experiments. Data represent the mean and standard deviation of 5-10 nail samples. The Q24 data for passive transport are the average of the 24-h data in the 24- and 94-h passive transport experiments.
Figure 2.
Fluxes (J) of ciclopirox across human nail plates in constant 9 V cathodal iontophoretic and 94-h passive transport experiments. Data represent the mean and standard deviation of 5-10 nail samples.
CIC Release from the Nail Plate
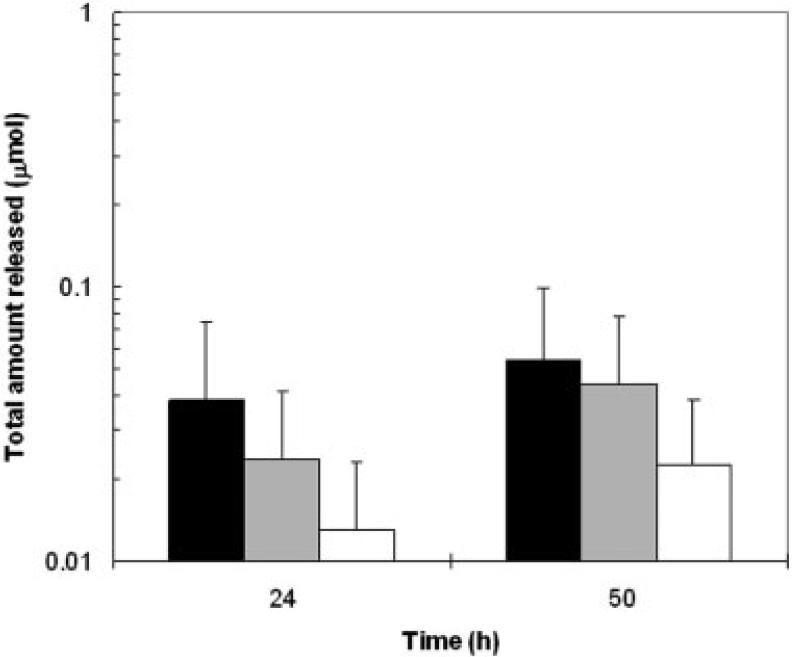
Figure 3 shows the amounts of CIC released into the receptor at 24 and 50 h from the nail after iontophoretic and passive applications. The amounts of CIC released from the nail into the receptor varied from 0.01-0.04 μmol (0.002-0.008 mg) on the first day after CIC administration and increased to 0.02-0.05 μmol (0.004-0.01 mg) the second day. The continuous release of CIC from the nail over time is consistent with the large amounts of CIC delivered into the nail forming a drug reservoir (see “CIC delivery into the nail plate”) under these conditions. No significant difference was found among the release data of the iontophoretic, passive, and Penlac® delivery (p>0.05). In addition, the amount of CIC released from the nail plate after iontophoresis was not significantly different from that delivered by Penlac® in 24 h, Q24 (p>0.05). The data suggest that the nail served as a drug reservoir and slowly released a significant amount of CIC in an extended manner after iontophoresis, which was not significantly different from that provided in the first 24 h after Penlac® application.
Figure 3.
Total amounts of ciclopirox released from the nail plates at 24 and 50 h after 9 V cathodal iontophoretic (dark bars), 24-h passive (gray bars), and Penlac® (open bars) delivery. Data represent the mean and standard deviation of 5-10 nail samples.
CIC Delivery into the Nail Plate
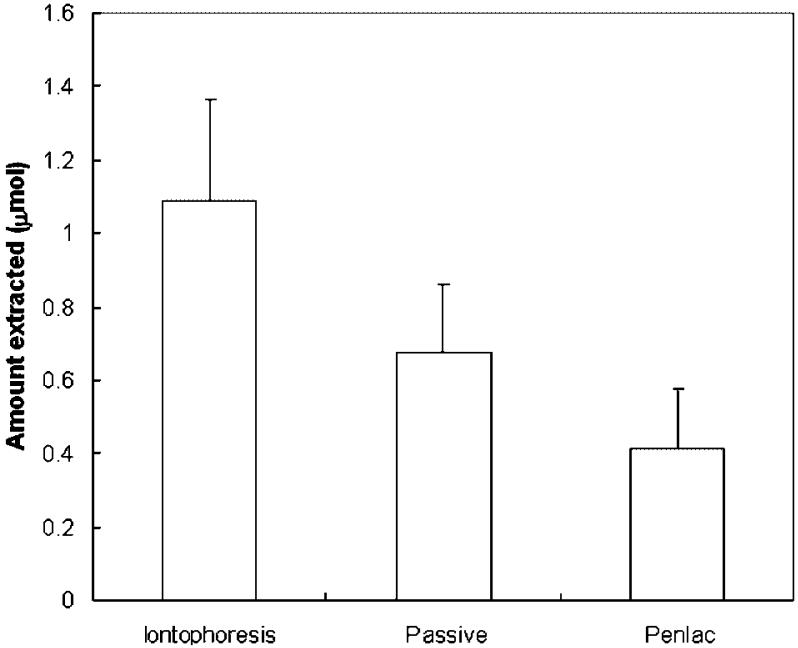
The results in the extraction studies showed substantial amounts of CIC remaining in the nails after the release experiments (Fig. 4). The amounts of CIC extracted from the nails at the end of the release experiments were 1.1 μmol (0.23 mg) for the iontophoresis group, 0.7 μmol (0.14 mg) for the 24-h passive group, and 0.4 μmol (0.08 mg) for the Penlac® group. These amounts represent less than 4% of the total dose applied onto the nails in the iontophoresis and Penlac® experiments. Cathodal iontophoresis significantly increased CIC loading into the nails compared to the 24-h passive delivery and Penlac® treatment (p<0.05). The drug loading after 24-h passive transport was approximately two times higher than that after Penlac® treatment (p<0.05). The concentrations of CIC in the nail plates were calculated by dividing the amounts of CIC extracted by the volume estimated from the effective diffusion area of 0.64 cm2 and average nail thickness of 0.6 mm. For iontophoretic delivery, the CIC concentration in the nails estimated using this method was 6 mg/mL. This value was significantly higher than the CIC minimum inhibitory concentrations (MIC) of 1-20 μg/mL for dermatophytic molds such as T. rubrum and T. mentagrophytes. The CIC concentration in the nails for Penlac® was 2 mg/mL. This value is in the same order of magnitude (~1 mg CIC/g nail, assuming nail density28,33 of 1.3 g/mL) as those found in previous studies,5,11,15 despite the different experimental designs, doses, and durations in the present and previous experiments. The large data variability observed in the present study was also similar to those in the previous studies.
Figure 4.
Amounts of ciclopirox extracted from the nail plates after constant 9 V cathodal iontophoretic, 24-h passive, and Penlac® transport and subsequent release experiments. Data represent the mean and standard deviation of 5-10 nail samples.
DISCUSSION
CIC is both fungistatic and fungicidal. Specifically, CIC chelates polyvalent cations resulting in the inhibition of the metal-dependent enzymes that are responsible for the degradation of peroxides within the fungal cell.34 CIC nail lacquer (Pelnac®) is applied daily for as many as 48 weeks. Repeated dosing for 1-2 weeks is usually required to achieve CIC concentrations in the deepest layers of the nail higher than its MIC.5 In the present study, constant voltage iontophoresis was shown to significantly enhance CIC penetration into and across the nails. The amounts of CIC delivered across the nail plate into the receptor increased from 0.005 mg after 3-h iontophoresis to 0.05 mg after 24-h iontophoresis. Assuming slow clearance and a tissue volume of 0.1 mL underneath the nail plate in vivo, the concentrations of CIC in the tissue at 3 and 24 h during iontophoretic delivery would be 0.05 and 0.5 mg/mL, respectively, above its MIC. Together with the high CIC concentration in the nails (6 mg/mL, also above the MIC) delivered by iontophoresis, the present results suggest the feasibility of iontophoretic delivery of CIC in nail antifungal treatment. Iontophoresis also shortened the apparent transport lag time compared to passive transport. This further suggests that the dosing time can be shortened in the iontophoretic treatment.
Although CIC penetration was enhanced by iontophoresis, the enhancement factor was significantly lower than that predicted from the Nernst-Planck equation at 9 V.25 The discrepancy between the experimental data and Nernst-Planck prediction and the low CIC transference may be related to the following. First, the low concentration of CIC in the donor (0.05 M) with respect to that of the electrolyte in the receptor (0.15 M PBS) could reduce the transference of CIC across the nail plate. The CIC anion has a larger molecular size and thus smaller electromobility than the electrolyte in the receptor solution. Significant ion competition from the receptor ions in iontophoresis is therefore anticipated. The effect of ion competition was also amplified by hindered transport25 in the partially hydrated nail; the partially hydrated nail due to the presence of ethanol in the donor is expected to have smaller effective pore size than the fully hydrated nail. Second, the unfavorable charge-charge interactions between the negatively charged nail plate and CIC are likely to reduce CIC transference during iontophoresis. The low CIC concentration in the donor (low ionic strength) could magnify the effects of the charge- charge interactions and decrease CIC partitioning into the negatively charged pathways in the nail. Third, the pH in the donor and receptor (pH gradient across the nail) could affect CIC iontophoretic transport. The degree of ionization of CIC (pKa of 7.2) at the receptor/nail interface and possibly in the nail was lower in the receptor (pH of 7.4) than that in the donor. The lower degree of CIC ionization would result in lower CIC iontophoretic transport. Fourth, the hydroxide ion (pH from 8 to 11) that is a by-product of the electrochemical reaction at the Pt electrode in the donor would compete with CIC transport across the nail plate. Despite the low hydroxide ion concentration, it has high electromobility and could reduce CIC transference particularly at the end of the iontophoresis experiments when the donor pH was high. Furthermore, the osmotic pressure gradient from the donor to the receptor would result in a convective solvent flow from the receptor to the donor. Although small, the electroosmotic flow was also from the receptor (anode) to the donor (cathode) under the present iontophoretic conditions. Both could hinder CIC transungual transport.
The present study has examined constant voltage transungual iontophoresis. These results have provided important information in the development of a portable transungual iontophoretic device such as an all-in-one disposable self-contained system composed of a disposable power source with low manufacturing cost. The device can be in the form of a thin, flexible patch with the battery embedded in the patch between the electrodes. When applied to the nail (on the finger or toe), the drug delivery electrode (donor electrode) is in contact with the nail surface, and the return electrode is in contact with skin away from the nail to complete the electric circuit. In the embedded battery patch design, the transungual iontophoresis system will not require a bulky external electric power source and inconvenient wiring between the fingernail or toenail and the power source. This allows the application of iontophoresis for an extended period of time on the nail and negates the necessity for the patient to be immobile during the application of iontophoresis.
The results in the present CIC release and extraction studies suggest that enhanced drug delivery into the nail plate is also important. When the nail is loaded with the drug from iontophoresis, the drug-loaded nail plate can serve as a drug depot for sustained release of the drug directly to the nail bed and surrounding tissues for prolonged nail treatment after the removal of the device. For example, the CIC device can be applied at night (preferably at bed time) and then be removed the next day. After removal of the device, the amount of CIC in the nail would be above its MIC and CIC would be slowly released from the nail to the nail bed and surrounding tissues. The next dose can be applied in a few days when CIC in the nail is depleted. This would greatly reduce drug administration frequency and thus improve patient compliance. It should be noted that the enhanced delivery observed in the present in vitro study may not correlate with enhanced therapeutic efficacy, and clinical testing is required to confirm the clinical performance of transungual iontophoretic treatment.
CONCLUSION
The present study has demonstrated the feasibility of using constant voltage iontophoresis to enhance transungual transport of an antifungal drug, ciclopirox, across human nail. The concentrations of CIC in the nail and delivered across the nail plate following iontophoretic transport were higher than its MIC. Iontophoresis rapidly delivered CIC across the nail (compared to passive delivery) and formed a drug depot in the nail from which CIC was slowly released. The shorter lag time and enhanced amount of CIC penetrated into and across the nail plate in the iontophoretic transport study suggest the possibility of reducing the treatment time and enhancing treatment efficacy using iontophoresis. The use of a portable alkaline battery power supply in transungual iontophoresis that can be eventually developed into a small disposable battery-driven iontophoretic device for transungual delivery was examined in the present study.
ACKNOWLEDGMENTS
This research was supported in part by NIH grant GM063559. The authors thank the financial support from Boehringer Ingelheim Cares Foundation on the thesis work of Kelly A. Smith.
REFERENCES
- 1.Murdan S. 1st meeting on topical drug delivery to the nail. Expert Opin Drug Deliv. 2007;4:453–455. doi: 10.1517/17425247.4.4.453. [DOI] [PubMed] [Google Scholar]
- 2.Piraccini BM, Iorizzo M, Antonucci A, Tosti A. Treatment of nail disorders. Therapy. 2004;1:159–167. [Google Scholar]
- 3.Drake LA, Patrick DL, Fleckman P, Andr J, Baran R, Haneke E, Sapede C, Tosti A. The impact of onychomycosis on quality of life: Development of an international onychomycosis-specific questionnaire to measure patient quality of life. J Am Acad Dermatol. 1999;41:189–196. doi: 10.1016/s0190-9622(99)70047-2. [DOI] [PubMed] [Google Scholar]
- 4.Ajit C, Suvannasankha A, Zaeri N, Munoz SJ. Terbinafine-associated hepatotoxicity. Am J Med Sci. 2003;325:292–295. doi: 10.1097/00000441-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Bohn M, Kraemer KT. Dermatopharmacology of ciclopirox nail lacquer topical solution 8% in the treatment of onychomycosis. J Am Acad Dermatol. 2000;43:S57–S69. doi: 10.1067/mjd.2000.109072. [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 2000;43:S70–S80. doi: 10.1067/mjd.2000.109071. [DOI] [PubMed] [Google Scholar]
- 7.Finch JJ, Warshaw EM. Toenail onychomycosis: Current and future treatment options. Dermatol Ther. 2007;20:31–46. doi: 10.1111/j.1529-8019.2007.00109.x. [DOI] [PubMed] [Google Scholar]
- 8.Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663–672. [PubMed] [Google Scholar]
- 9.Neubert RH, Gensbugel C, Jackel A, Wartewig S. Different physicochemical properties of antimycotic agents are relevant for penetration into and through human nails. Pharmazie. 2006;61:604–607. [PubMed] [Google Scholar]
- 10.Kobayashi Y, Komatsu T, Sumi M, Numajiri S, Miyamoto M, Kobayashi D, Sugibayashi K, Morimoto Y. In vitro permeation of several drugs through the human nail plate: Relationship between physicochemical properties and nail permeability of drugs. Eur J Pharm Sci. 2004;21:471–477. doi: 10.1016/j.ejps.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Hui X, Baker SJ, Wester RC, Barbadillo S, Cashmore AK, Sanders V, Hold KM, Akama T, Zhang YK, Plattner JJ, Maibach HI. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96:2622–2631. doi: 10.1002/jps.20901. [DOI] [PubMed] [Google Scholar]
- 12.Hui X, Chan TC, Barbadillo S, Lee C, Maibach HI, Wester RC. Enhanced econazole penetration into human nail by 2-n-nonyl-1,3-dioxolane. J Pharm Sci. 2003;92:142–148. doi: 10.1002/jps.10291. [DOI] [PubMed] [Google Scholar]
- 13.Khengar RH, Jones SA, Turner RB, Forbes B, Brown MB. Nail swelling as a pre-formulation screen for the selection and optimisation of ungual penetration enhancers. Pharm Res. 2007;24:2207–2212. doi: 10.1007/s11095-007-9368-3. [DOI] [PubMed] [Google Scholar]
- 14.Murdan S. Drug delivery to the nail following topical application. Int J Pharm. 2002;236:1–26. doi: 10.1016/s0378-5173(01)00989-9. [DOI] [PubMed] [Google Scholar]
- 15.Hui X, Wester RC, Barbadillo S, Lee C, Patel B, Wortzmman M, Gans EH, Maibach HI. Ciclopirox delivery into the human nail plate. J Pharm Sci. 2004;93:2545–2548. doi: 10.1002/jps.20159. [DOI] [PubMed] [Google Scholar]
- 16.Monti D, Saccomani L, Chetoni P, Burgalassi S, Saettone MF, Mailland F. In vitro transungual permeation of ciclopirox from a hydroxypropyl chitosan-based, water-soluble nail lacquer. Drug Dev Ind Pharm. 2005;31:11–17. doi: 10.1081/ddc-43935. [DOI] [PubMed] [Google Scholar]
- 17.Kalinowski DP, Edsberg LE, Hewson RA, Johnson RH, Brogan MS. Low-voltage direct current as a fungicidal agent for treating onychomycosis. J Am Podiatr Med Assoc. 2004;94:565–572. doi: 10.7547/0940565. [DOI] [PubMed] [Google Scholar]
- 18.Kasting GB. Theoretical models for iontophoretic delivery. Adv Drug Deliv Rev. 1992;9:177–199. [Google Scholar]
- 19.Pikal MJ. The role of electroosmotic flow in transdermal iontophoresis. Adv Drug Deliv Rev. 1992;9:201–237. doi: 10.1016/s0169-409x(00)00138-1. [DOI] [PubMed] [Google Scholar]
- 20.Chizmadzhev YA, Indenbom AV, Kuzmin PI, Galichenko SV, Weaver JC, Potts RO. Electrical properties of skin at moderate voltages: Contribution of appendageal macropores. Biophys J. 1998;74:843–856. doi: 10.1016/S0006-3495(98)74008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaser RW, Leikin SL, Chernomordik LV, Pastushenko VF, Sokirko AI. Reversible electrical breakdown of lipid bilayers: Formation and evolution of pores. Biochim Biophys Acta. 1988;940:275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- 22.James MP, Graham RM, English J. Percutaneous iontophoresis of prednisolone—A pharmaco-kinetic study. Clin Exp Dermatol. 1986;11:54–61. doi: 10.1111/j.1365-2230.1986.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 23.Murthy SN, Wiskirchen DE, Bowers CP. Iontophoretic drug delivery across human nail. J Pharm Sci. 2007;96:305–311. doi: 10.1002/jps.20757. [DOI] [PubMed] [Google Scholar]
- 24.Murthy SN, Waddell DC, Shivakumar HN, Balaji A, Bowers CP. Iontophoretic permselective property of human nail. J Dermatol Sci. 2007;46:150–152. doi: 10.1016/j.jdermsci.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Hao J, Li SK. Transungual iontophoretic transport of polar neutral and positively charged model permeants: Effects of electrophoresis and electroosmosis. J Pharm Sci. 2008;97:893–905. doi: 10.1002/jps.21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao J, Li SK. Mechanistic study of electroosmotic transport across hydrated nail plates: Effects of pH and ionic strength. J Pharm Sci. 2008;97:5186–5197. doi: 10.1002/jps.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao J, Smith KA, Li SK. Chemical method to enhance transungual transport and iontophoresis efficiency. Int J Pharm. 2008;357:61–69. doi: 10.1016/j.ijpharm.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunt HB, Miller MA, Kasting GB. Water diffusivity in human nail plate. J Pharm Sci. 2007;96:3352–3362. doi: 10.1002/jps.20988. [DOI] [PubMed] [Google Scholar]
- 29.Jemec GB, Agner T, Serup J. Transonychial water loss: Relation to sex, age and nail-plate thickness. Br J Dermatol. 1989;121:443–446. doi: 10.1111/j.1365-2133.1989.tb15511.x. [DOI] [PubMed] [Google Scholar]
- 30.Nuutinen J, Harvima I, Lahtinen MR, Lahtinen T. Water loss through the lip, nail, eyelid skin, scalp skin and axillary skin measured with a closed-chamber evaporation principle. Br J Dermatol. 2003;148:839–841. doi: 10.1046/j.1365-2133.2003.05257.x. [DOI] [PubMed] [Google Scholar]
- 31.Murdan S, Hinsu D, Guimier M. A few aspects of transonychial water loss (TOWL): Inter-individual, and intra-individual inter-finger, inter-hand and inter-day variabilities, and the influence of nail plate hydration, filing and varnish. Eur J Pharm Biopharm. 2008;70:684–689. doi: 10.1016/j.ejpb.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Challapalli PV, Stinchcomb AL. In vitro experiment optimization for measuring tetrahydrocannabinol skin permeation. Int J Pharm. 2002;241:329–339. doi: 10.1016/s0378-5173(02)00262-4. [DOI] [PubMed] [Google Scholar]
- 33.Gunt HB, Kasting GB. Effect of hydration on the permeation of ketoconazole through human nail plate in vitro. Eur J Pharm Sci. 2007;32:254–260. doi: 10.1016/j.ejps.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Gupta AK. Ciclopirox: An overview. Int J Dermatol. 2001;40:305–310. doi: 10.1046/j.1365-4362.2001.01156.x. [DOI] [PubMed] [Google Scholar]