Abstract
Recent epidemiologic studies have suggested that serum dehydroepiandrosterone sulfate (DHEAS) levels have a significant inverse correlation with the incidence of cardiovascular diseases. However, direct evidence for the association with DHEAS and vascular disorders has not yet been explored. DHEAS significantly reduced neointima formation 28 days after surgery without altering other serum metabolite levels in a rabbit carotid balloon injury model. Immunohistochemical analyses revealed the reduction of proliferating cell nuclear antigen (PCNA) index and increase of TdT-mediated dUTP-biotin Nick End Labeling (TUNEL) index, expressing differentiated vascular smooth muscle cell (VSMC) markers in the media 7 days after surgery. In vitro, DHEAS exhibited inhibitory effects on VSMC proliferation and migration activities, inducing G1 cell cycle arrest with upregulation of one of the cyclin dependent kinase (CDK) inhibitors p16INK4a and apoptosis with activating peroxisome proliferator-activated receptor (PPAR)-α in VSMCs. DHEAS inhibits vascular remodeling reducing neointima formation after vascular injury via its effects on VSMC phenotypic modulation, functions and apoptosis upregulating p16INK4a/activating PPARα. DHEAS may play a pathophysiological role for vascular remodeling in cardiovascular disease.
Keywords: hormones, restenosis, vascular smooth muscle cell, apoptosis
1. Introduction
Dehydroepiandrosterone (DHEA) and its sulfate ester (DHEAS) are the most abundant circulating adrenal steroids in the human body. However, other than their role as precursors to androgens and estrogens, their physiological effects are unknown. Plasma DHEAS levels decline linearly with age1, 2 and this decline has led to the suggestion that DHEAS can be implicated in the process of aging, e.g., atherosclerosis. Observational studies have indicated that plasma DHEAS is inversely correlated with cardiovascular diseases (CVD) or angiographically defined coronary artery diseases in men.3–5 These studies have suggested that DHEAS may have an anti-atherosclerotic or –arteriosclerotic effect.
Several reports have indicated that DHEA administration reduces atherosclerotic lesions significantly in hypercholesterolemic rabbits.6–9 In these studies, however, since significant lipid profile changes were not detected after DHEA administration, the mechanism of the anti-atherosclerotic effect by DHEA remains unknown and no follow-up studies have been performed. We focused on DHEAS effects on the biology of VSMCs because VSMC accumulation into the intima and their activation are hallmarks of not only atherosclerosis10, 11 but also in-stent restenosis, which is still a major problem after arterial stent implantation.12 Although we previously reported that DHEAS inhibits proliferation and migration of VSMCs in vitro,13 in vivo studies focused on DHEAS effects on VSMCs have never been performed.
In addition, several lines of evidence have suggested that DHEAS is not just a reservoir of DHEA: 1) DHEAS, but not DHEA, can activate peroxisome proliferator-activated receptor (PPAR)-α;14 2) DHEAS has a more potent inhibitory effect on VSMC migration than DHEA in rabbit VSMC cell lines (SM-3);13 3) DHEAS reduces chronic hypoxic pulmonary hypertension in rats; 15 and 4) DHEAS inhibits vascular inflammation. 16 Until now, however, the effect of DHEAS on arterial remodeling following balloon injury had never been studied.
In the present study, to test the hypothesis that DHEAS has an inhibitory effect on neointima formation, we examined exogenous DHEAS effects in a rabbit carotid balloon injury model. We also assessed in vitro effects of DHEAS on VSMC functions, and attempted to elucidate the mechanism for the biological effect of DHEAS in vascular biology.
2. Materials and methods
2.1. Animal Study
All animal studies were performed with the approval of the Osaka Medical College Animal Care and Use Committee. Adult male Japanese white rabbits (2.5 to 3.0 kg) fed with normal chow were assigned to two groups: DHEAS-treated (DHEAS) group and saline-treated (control) group. DHEAS (Mylis™, Kanebo Pharmaceutical, Japan) was administered intravenously (20 mg/kg/day) via an ear vein from 3 days before surgery until sacrifice, and saline was administered as a control vehicle. Carotid balloon injury was performed as described previously.17 The carotid arteries were examined histologically and blood samples were collected for serological analysis at each time point. (supplemental information)
2.2 Immunohistochemistry
Immunohistochemistry was performed as described previously.18 Brief procedure is described in supplemental information. Primary antibodies (supplemental information) were visualized with Cy2 or Cy3 conjugated anti-goat or rabbit IgG (Jackson ImmunoResearch; 1:500) followed by nuclear counter staining with 4′,6-diamdino-2-phenylindole (DAPI).
2.3. Proliferation and Apoptosis assay in the Media
Medial proliferating and apoptotic cells were detected by immunostaining for PCNA and TUNEL using in situ cell death detection kit (Roche) according to the manufacturer’s instructions, respectively. After the staining, PCNA- or TUNEL-positive and -negative nuclei were counted in 3 different HPF (200X) per section. Proliferation and apoptosis activity was expressed as the PCNA and TUNEL labeling index calculated by dividing positive labeled cells by total cell number, respectively.
2.4. Western Blot Analysis
Western blot analysis was performed as described previously.18 Brief procedure is described in supplemental information.
2.5. Cell culture
Rabbit carotid VSMCs were obtained by an explant method, as previously described.17 Cells were passaged by trypsinization, and used for the experiment between passages 3 and 5.
2.6. Cell Proliferation and Migration Assay
VSMC proliferation was assessed by BrdU incorporation as described previously.18 VSMC migration was assessed by the modified Boyden’s chamber method, as described previously.17 Brief procedure is described in supplemental information.
2.7. Flow Cytometry Analysis
For cell cycle analysis, flow cytometry was performed as described previously.18 Brief procedure is described in supplemental information.
2.8 Apoptosis Assay
VSMCs (3×104 cells/well) were cultured in 5% FCS/MEM in 4 well-chamber slides (Nunc) at 37°C, and incubated with DHA at a concentration of 0, 10−7, 10−6 or 10−5 mol/L for 24 hours under serum starved condition (0.5% FCS) in the presence of DHEAS (10−5 mol/L) or not. For the PPARα deletion study, human PPARα siRNA (Santa Cruz) was transfected with human aortic VSMCs (ATCC) and incubated for 3 days and TUNEL staining was performed. TUNEL-positive and total cells were counted in 5 different HPF (200X), and apoptosis was evaluated as the percentage of TUNEL-positive cells to total cell number in each chamber.
2.9. Statistical analysis
All values were expressed as mean ± SEM. Statistical analyses were performed using statistical software (Statview™). Mann-Whitney U-test was used for the comparison between two groups and ANOVA was used followed by post-hoc test with a Tukey procedure for multiple groups. A value of P<0.05 was considered significant.
3. Experimental results
3.1. Serum Cholesterol Levels, Triglyceride, DHEAS, DHEA, Estradiol and Testosterone in Rabbits (Table)
Table.
Serum Levels of Total Cholesterol (T-Chol), Triglyceride (TG), DHEA, DHEAS, Estradiol and Testosterone (TS) following DHEAS Administration
| Duration of DHEAS Administration (n=8) |
|||||
|---|---|---|---|---|---|
| 0 day | 3 days | 7 days | 14 days | 28 days | |
| T-Chol (mg/dl) | 35±4 | 46±3NS | 58±8NS | 61±12NS | 36±4NS |
| TG (mg/dl) | 59±13 | 48±2NS | 70±10NS | 61±6NS | 94±21NS |
| DHEA (ng/ml) | 0.50±0.2 | 0.28±0.08NS | 0.43±0.14NS | 0.39±0.07NS | 0.39±0.07NS |
| DHEAS (ng/ml) | < 20 | 42±6* | 1408±453* | 1123±448* | 81±11* |
| Estradiol (pg/ml) | 8.4±0.6 | 7.3±0.8NS | 7.1±0.6NS | 7.2±0.5NS | 7.7±0.6NS |
| TS (ng/ml) | 242±44 | 410±74NS | 296±32NS | 219±29NS | 206±19NS |
Values are presented as mean±SEM.
, P<0.0001 and
vs. 0 day (control).
The serum level of DHEAS before treatment was under the detection limit (<20 ng/ml) of the assay. Daily administration of DHEAS (20 mg/kg) resulted in significant increases in the serum DHEAS levels at 7 days to 1408±453 ng/ml and 14 days to 1123±448 ng/ml (physiological doses in human), which then decreased at 28 days to 81±11 ng/ml. All the other hormone and lipid levels that might be affected by conversion of DHEAS did not change significantly, even in the setting of robust serum DHEAS elevation.
3.2. Effect of DHEAS on Neointima Formation and Medial Cell Proliferation
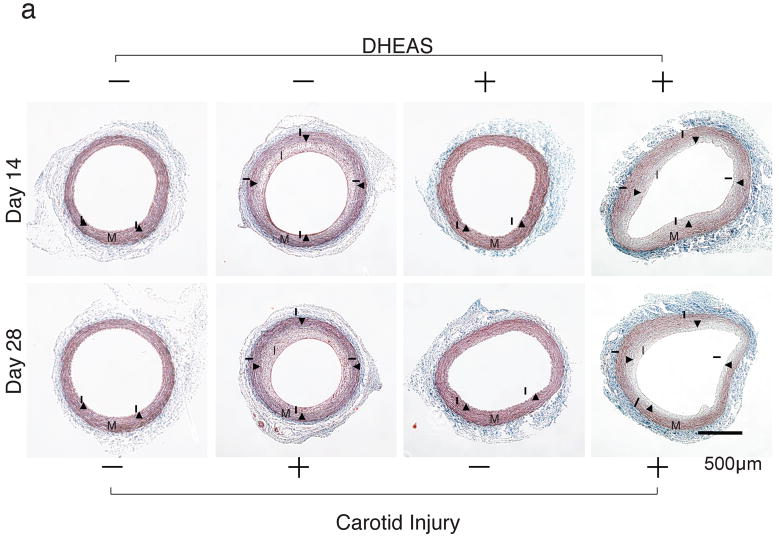
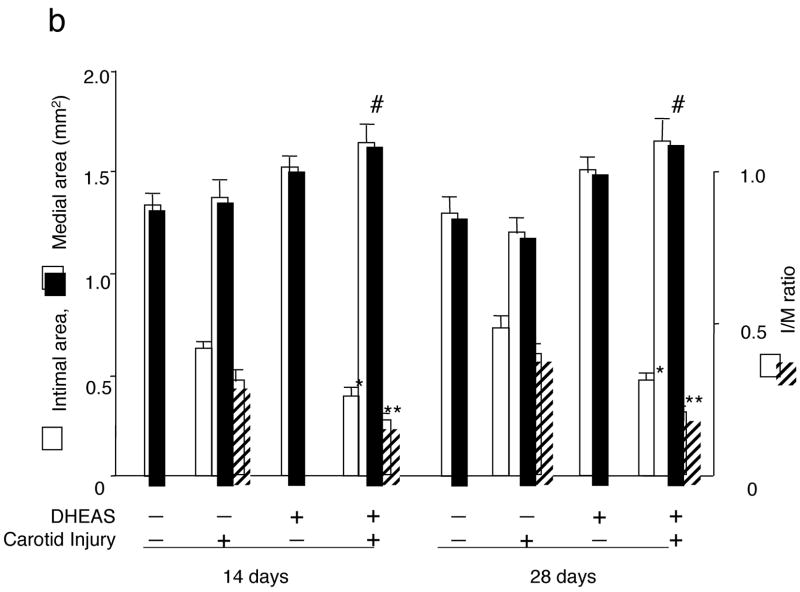
DHEAS inhibited neointima formation 14 days after balloon injury in carotid arteries (Figure 1a and b). The intimal area and I/M ratio in the DHEAS group (n=10) were significantly reduced compared to the control group (n=8) at 14 days after carotid injury (0.37±0.05 mm2 and 0.22±0.02 versus 0.64±0.02 mm2 and 0.48±0.04, p=0.0002 and <0.0001, respectively). A similar inhibitory effect of neointima formation by DHEAS was also observed at 28 days after surgery (0.48±0.03 mm2 and 0.29±0.01 versus 0.73±0.05 mm2 and 0.61±0.03, p=0.0006; n=8 and <0.0001; n=7, respectively). There was also a difference in the medial area between the control group and DHEAS group with a thicker medial layer in the DHEAS group (1.39±0.08 versus 1.64±0.10 mm2 at 14 days, p=0.09838 and 1.20±0.07 versus 1.66±0.09 mm2 at 28 days, p=0.000922) (Figure 1c).
Figure 1. DHEAS Reduces Neointima Formation Following Carotid Balloon Injury.
a, The entire cross-sections of Masson’s Trichrome-stained sections from balloon injured carotid arteries. I, the intima; M, media; and arrow-heads, internal elastic lamina (IEL). b, Morphometric analysis. Comparisons of intimal, medial area, and intima/media ratio (I/M) between the control group and the DHEAS group. * and **, P<0.01 and #, P<0.0001 at 14 and 28 days vs. control.
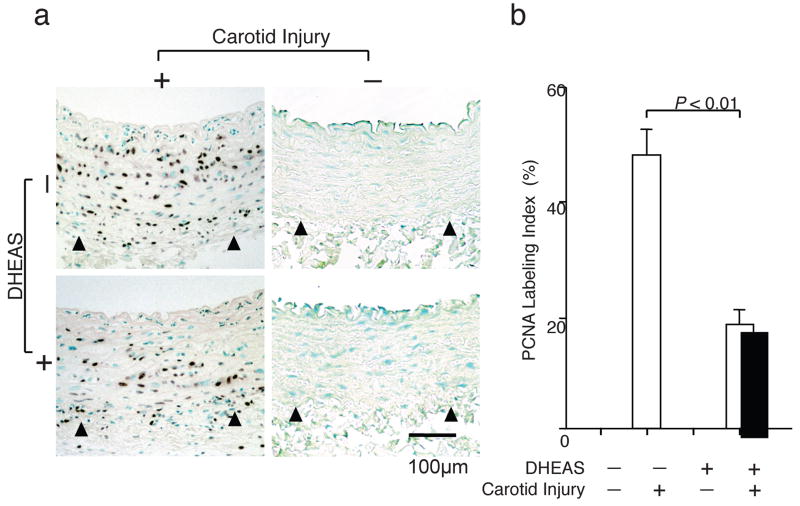
DHEAS also significantly suppressed medial cell proliferation (Figure 2a) as indicated by a reduced PCNA labeling index in the media 7 days after carotid injury. (45.8±2.9% in control; n=5, 17.8±1.7% in the DHEAS group; n=5, p=0.01116) (Figure 2b)
Figure 2. DHEAS Reduces Medial Cell Proliferation Activity.
Immunostaining for PCNA to evaluate proliferation activity in injured arteries. a, control group and DHEAS group. PCNA-positive nuclei are stained in black and arrow-heads indicate the external elastic lamina (EEL). Nuclei are stained with methylgreen in green. b, PCNA labeling index as a percentage of PCNA-positive nuclei per total nuclei in the media.
3.3. Effect of DHEAS on Phenotypic Modulation of VSMCs after Vascular Injury
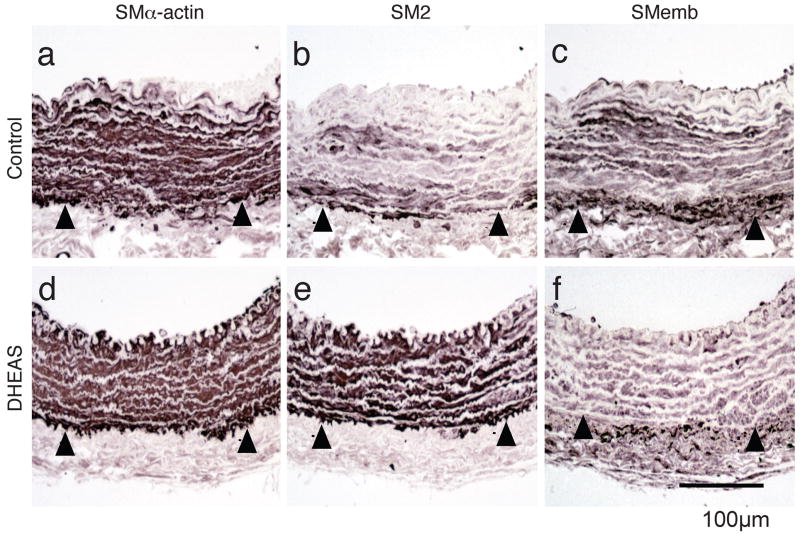
VSMC phenotypic modulation in injured carotid arteries by DHEAS was evaluated immunohistochemically using the following various markers: smooth muscle (SM) α-actin for a universal marker of VSMC; SM2 (smooth muscle specific myosin heavy chain) for a differentiated phenotype marker of VSMC; and SMemb (non-muscle myosin heavy chain) for a dedifferentiated phenotype marker of VSMC.19, 20 There was no difference in the medial expression of SMα-actin after injury between the control and the DHEAS groups (Figure 3a and d). The medial expression of SM2 was strikingly reduced in the control group compared with the DHEAS group (Figure 3b and e). In contrast, the medial expression of SMemb was more prominent in the control group than the DHEAS group (Figure 3c and f). The similar SM2 and SMemb expression differences were observed in both media and neointima at day 14 after carotid injury. (supplemental Figure Ia–d)
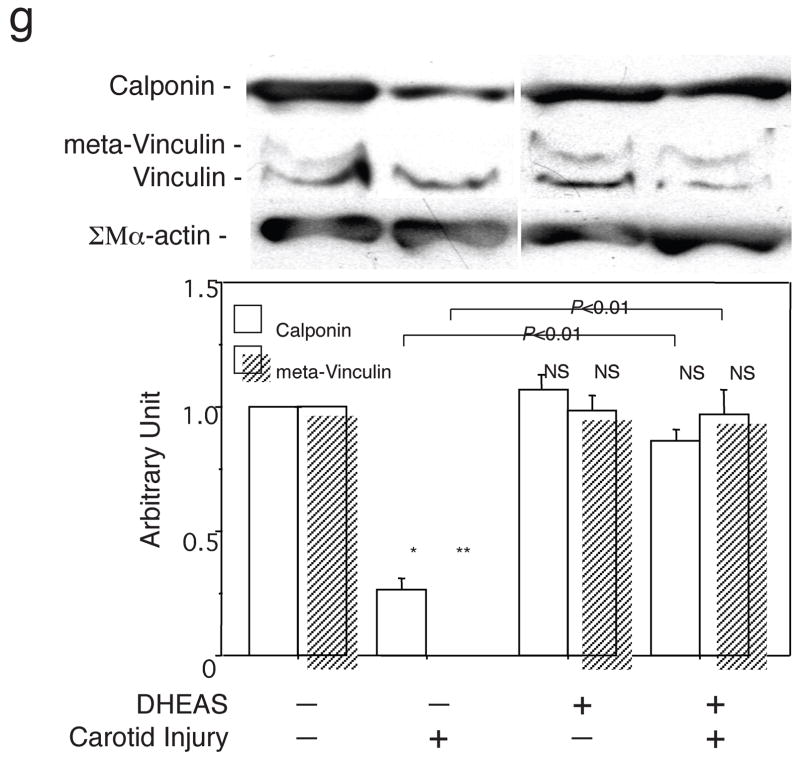
Figure 3. DEHAS Modulates Medial VSMC Phenotype Following Carotid Balloon Injury.
Immunostaining for SMα-actin (a and d), SM2 (b and e), and SMemb (c and f) in injured arteries. a, b and c: control group; d, e and f: DHEAS group. The positive expressions of each marker are stained in black. Arrow-heads indicate EEL. g, Western analyses for SMα-actin (42 kD), calponin (34 kD), meta-vinculin (150 kD), and vinculin (130 kD) in the control group and the DHEAS group with or without balloon injury. Immunoblots are shown with densitometrical analysis. * and **, P<0.0001 and NS vs. (DHEAS: -, Carotid Injury: -).
Western blot analysis also demonstrated remarkable decreases in calponin and meta-vinculin levels (relative value to non-injured artery; calponin, 0.27±0.04 vs. 1.00±0.00 and meta-vinculin, 0.00±0.00 vs. 1.00±0.00, p<0.0001) in injured arteries compared with non-injured artery. Downregulation of these marker expressions was significantly reversed by DHEAS. (calponin, 0.82±0.03 vs. 0.27±0.04 and meta-vinculin, 0.87±0.12 vs. 0.00±0.00, p<0.0001) (Figure 3g)
3.4. Effects of DHEAS on VSMC Proliferation, Migration and Cell Cycle In Vitro
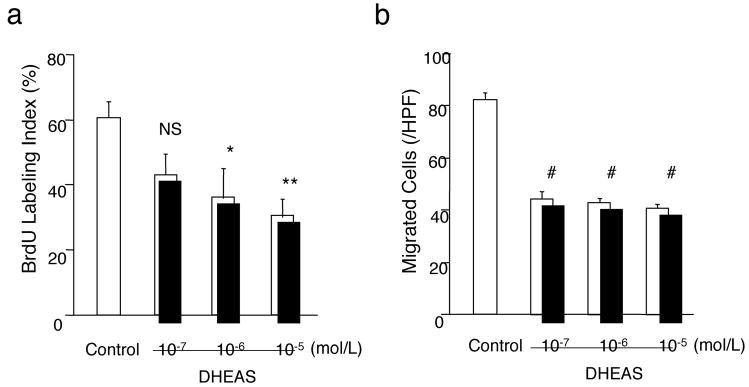
After 24 hours incubation of cultured VSMCs stimulated by 10% FCS/MEM with DHEAS, BrdU labeling index was reduced dose-dependently by a maximum of 50% (DHEAS) at a concentration of 10−5 mol/L compared with control (Figure 4a). In migration assay, co-incubation with DHEAS for 3 hours decreased PDGF-induced VSMC migration by a maximum of 52% (DHEAS) at a concentration of 10−5 mol/L compared to the control (Figure 4b). Cell cycle analysis by flow cytometry also demonstrated that DHEAS induced G1 cell cycle arrest in proliferating VSMCs. (supplemental Figure II)
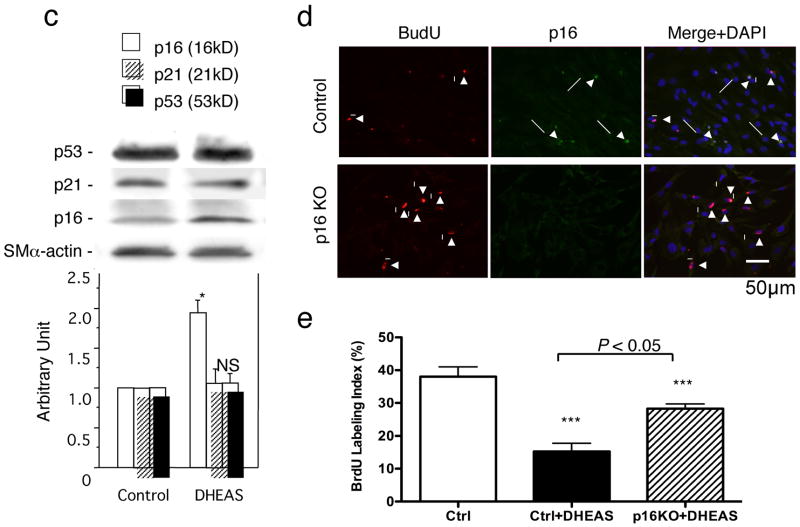
Figure 4. DHEAS Inhibits VSMC Proliferation and Migration Inducing p16INK4a.
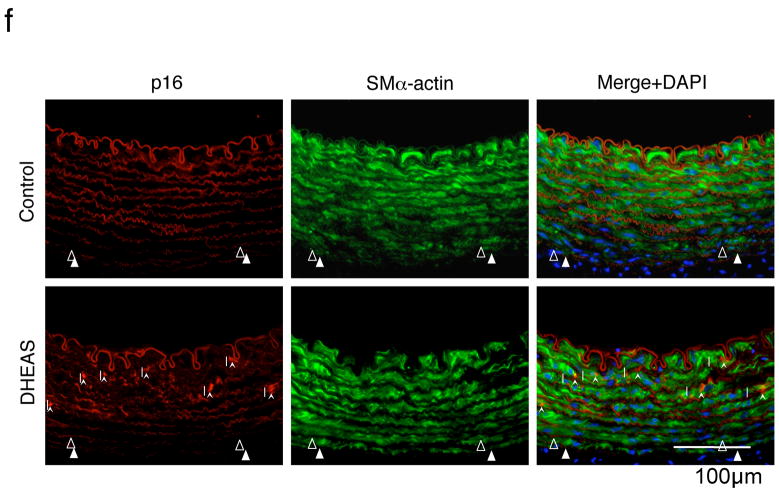
a and e, Proliferation activity evaluated as a percentage of BrdU positive cells. b, Migration activity expressed as the number of migrated cells/HPF (200X). Control indicates the each assay in the absence of DHEAS. *, P<0.05; **, P<0.01; ***, P<0.05: and #, P<0.0001 vs. control (ctrl). c, Western blot analyses of CDK inhibitors, p16, p21, and p53 in VSMCs. Immunoblots are shown with densitometrical analysis. *, P<0.01 and NS vs. control. d, Double immunofluorescent staining: Red, BrdU, Green, p16INK4a, and Blue, nuclei (DAPI). Arrows, p16INK4a positive cells and Arrow-heads, BrdU-positive cells. e, Proliferation activity was expressed as BrdU labeling index in control and p16 knockout (KO) VSMCs with or without DHEAS (10 μmol/L) f, Double immunofluorescent staining in injured arteries. Red, p16; Green, SMα-actin; Blue, nuclei (DAPI). Arrow-heads indicate EEL and arrows indicate p16-positive cells.
3.5. DHEAS upregulates CDK inhibitor, p16INK4a, but not p21Cip1 and p53
To further explore a certain molecular mechanism for the inhibitory effect of DHEAS on VSMC proliferation/cell cycle, we examined the expression of CDK inhibitors in VSMCs. VSMCs were collected after 18 hours co-incubation with DHEAS (10 μmol/L) or PBS (control) in 5% FCS/MEM following 48 hours serum starvation for Western blot analysis. Although the expression of p21Cip1 and p53 were not changed (p21Cip1; 1.01±0.06 vs. 1.00±0.00, p=0.9876 and p53; 1.07±0.05 vs. 1.00±0.00, p=0.9866), p16INK4a was significantly upregulated by DHEAS compared with control (1.86±0.17 vs. 1.00±0.00, p=0.0018). (Figure 4c) In addition, in vitro p16INK4a knockout study exhibited reversed anti-proliferation of VSMCs by DHEAS treatment. (p16KO+DHEAS; 28.3±1.5 vs. Ctrl+DHEAS; 15.3±2.5 %, p<0.05) (Figure 4d and e) Indeed, striking medial p16INK4a expressions were observed only in the DHEAS-treated injured arteries 3 days after surgery. The merged image exhibits co-localization of p16INK4a and SMα-actin positive cells in the DHEAS-treated group. (Figure 4f)
3.6. Effects of DHEAS on VSMC Apoptosis
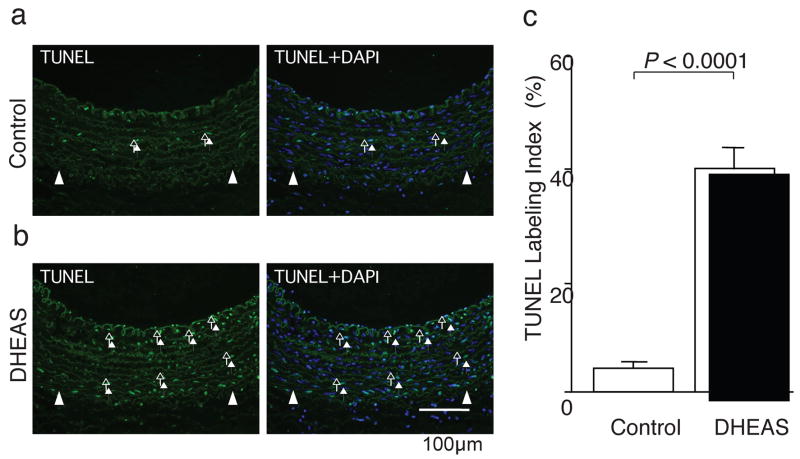
We evaluated the effects of DHEAS on medial cell apoptosis/necrosis by TUNEL staining (Figure 5a and b) 7 days after carotid injury. TUNEL labeling index was significantly greater in the DHEAS group than the control group (4.1±0.3% in control; n=5, 40.8±6.2% in the DHEAS group; n=5, p=0.01116). (Figure 5c) Although there was no remarkable difference of TUNEL positivity in the media, a number of TUNEL positive cells were observed in the neointima only in the DHEAS group 14 days after carotid injury. (supplemental Figure Ie)
Figure 5. DHEAS Induces Medical Cell (VSMC) Apoptosis Activating PPARα.
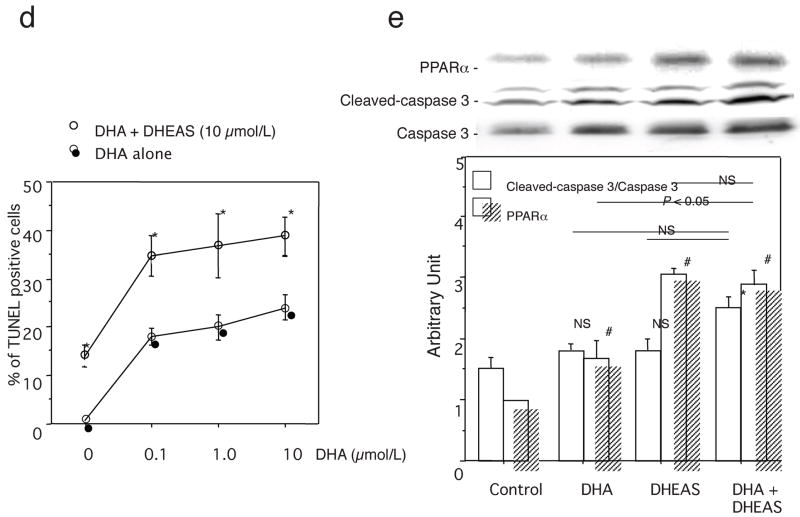
Fluorescent TUNEL staining in injured arteries: a, control group and b, DHEAS group. Arrows and arrow-heads indicate TUNEL-positive cells (green) and EEL, respectively. Blue: uclei with DAPI. c and d, TUNEL labeling index. *, P<0.01 vs. control and DHA treatment alone. e, Western analyses of caspase 3 (35 kD), cleaved-caspase 3 (17/19 kD) and PPARα (52 kD) in VSMCs after 18 hours serum starvation with DHA (10 μmol/L), DHEAS (10 μmol/L) and both of DHA and DHEAS or not (control). Immunoblots are shown with densitometrical analysis. *, P<0.05 and #, P<0.01 vs. control.
Next, PPARα ligand (DHA)-induced VSMC apoptosis was evaluated by TUNEL staining in the presence or absence of DHEAS (10 μmol/L) in vitro. DHA increased the percent of TUNEL-positive cells dose-dependently. On the other hand, DHEAS not only induced VSMC apoptosis by itself (14.3±2.3 vs. 0.7±0.3%, p=0.07652) but also co-incubation of DHEAS and DHA resulted in the greater extent of apoptosis in VSMCs at any concentration of DHA (0.1 μmol/L; 35.2±4.2 vs. 17.9±1.7%, p=0.0564, 1 μmol/L; 37.3±6.6 vs. 20.1±2.6%, p=0.0859, and 10 μmol/L; 39.1±4.0 vs. 24.1±2.6%, p=0.126). (Figure 5d) Although cleaved caspase 3/total caspase 3 ratio (DHA; 1.9±0.2 and DHEAS; 1.9±0.1 vs. 1.5±0.2 in control, p=0.9995) was limited by 25% by DHEAS without significant difference, co-incubation with DHA and DHEAS in VSMCs exhibited a significantly elevated cleaved-caspase 3/total caspase 3 ratio compared with the control (DHA+DHEAS; 2.3±0.1 vs. 1.5±0.2 in control, p=0.0136). (Figure 5e) The PPARα expression by DHEAS was higher than that by DHA, however, no significant further upregulation of PPARα was observed by the co-incubation compared with either DHA or DHEAS treatment alone (DHA+DHEAS; 2.9±0.3 vs. DHA; 2.2±0.3, p=0.2535 and DHEAS; 3.1±0.1, p=0.2196), (Figure 5e)
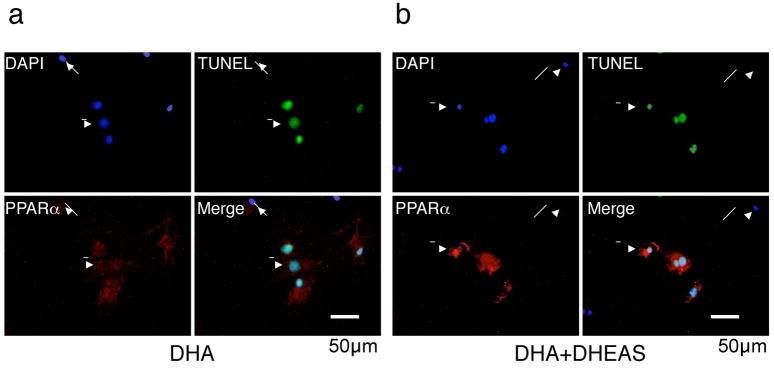
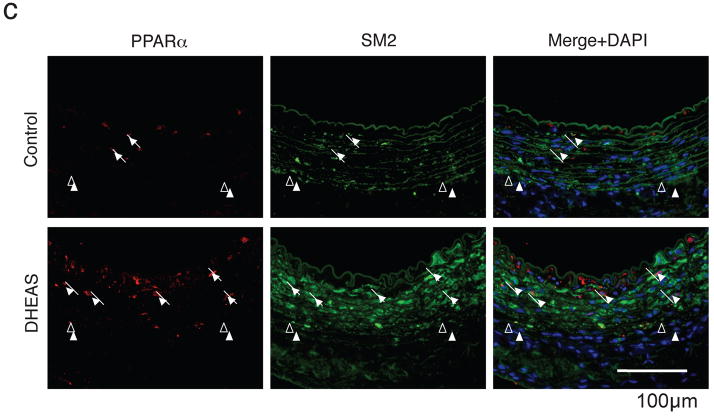
Immunocytochemical analysis showed that only TUNEL-positive cells co-expressed PPARα by DHA treatment alone, (Figure 6a) and co-treatment of DHA with DHEAS showed much higher expression of PPARα in only TUNEL-positive cells. (Figure 6b) Striking PPARα expression was also observed only in the DHEAS-treated group, and most of the expression was co-localized with SM2, indicating that PPARα was upregulated in injured medial VSMCs. (Figure 6c) In contrast, when the PPARα mRNA expression is silenced by siRNA technique in VSMCs, DHEAS-induced apoptosis was partially cancelled in consistent with the decreased expression of PPARα. (supplemental Figure III)
Figure 6. DHEAS Induces PPARα Expression in VSMCs and Injured Artery.
Cells were cultured in 0.5% FCS medium with DHA (10 μmol/L) in the presence (b) or absence (a) of DHEAS (10 μmol/L) for 24 hours. Double immunofluorescent staining: Red, PPARα, Green, TUNEL, and Blue, nuclei (DAPI). Arrows, TUNEL negative cells and Arrow-heads, TUNEL-positive cells. c, Injured carotid arteries in the control and the DHEAS group. Red, PPARα; Green, SM2; Arrows, PPARα-positive cells, and arrow-heads, EEL.
4. Discussion
In the present study, we first report the biological effect of DHEAS on neointima formation following arterial injury in a non-cholesterol fed rabbit carotid injury model which allow us to focus on VSMC biology without inflammatory response. DHEAS significantly inhibited neointima formation after balloon injury suppressing VSMC migration and proliferation activities. This was associated with upregulation of CDK inhibitor p16INK4a, and with induction of medial cell death/apoptosis through PPARα-involved signaling.
Differing from the estradiol-NO dependent indirect inhibitory effect of DHEA on atherosclerosis,9 the present study assumed the remote possibility that the inhibitory effect of DHEAS for neointima formation is due to its conversion to the other metabolites, because the serum levels of other hormones including metabolites of DHEAS were not changed significantly after DHEAS administration to rabbits. (Table). Indeed, our in vitro data shows direct inhibitory effects of DHEAS on VSMCs supporting the results of serological examination. Since there are no reports in which DHEAS-sulfatase, which can convert DHEAS to DHEA, activation is demonstrated in rabbits and evidences of differential DHEAS-sulfatase activity among species,21 we speculate that the significant decrease of serum DHEAS level at day 28 was a result from DHEAS metabolism by unknown enzymatic system into other metabolites but not DHEA or estradiol.
Interestingly, the medial area at 14 and 28 days after injury was significantly increased in the DHEAS-treated injured arteries (Figure 1c) inhibiting neointima formation by DHEAS. One possible reason for this phenomenon is an increase of extracellular matrices, because it has already been reported that DHEAS promotes collagenase22 and hyaluronate23 synthesis in rabbit fibroblasts. Indeed, Picro-Sirius red staining demonstrated the increased medial matrix including collagen fibers (stained in red) in the DEHAS-treated injured arteries. (supplemental Figure IV) We speculate that the interstitial space after medial cell apoptosis was replaced with the extracellular matrices produced from resident medial fibroblasts by DHEAS treatment.
Several lines of evidence have shown that phenotypic modulation is an important phenomenon in VSMC activation.10, 11 We have shown that DHEAS prevented the phenotypic change of VSMC from differentiated “contractile” phenotype to dedifferentiated “synthetic” phenotype that is normally induced by balloon injury as well as insulin-like growth factor 1 (IGF-1)24 and all-trans-retinoic acids (atRA).25 Recent studies have reported that PPARα activators (fibrate drugs) and its ligand (fish oil; DHA) could inhibit VSMC activation26 and induce apoptosis via p38 MAP kinase activation27, respectively. Our in vitro data have also demonstrated that DHEAS induced G1-cell cycle arrest in VSMCs (supplemental figure II) upregulating p16INK4a under serum-stimulating conditions but not under no serum condition and finally apoptosis activating caspase 3 along with PPARα expression, indicating that DHEAS-PPARα signaling pathway might be involved in the inhibitory/pro-apoptotic effect on proliferating VSMCs. However, since the activation of caspase 3 by DHEAS was limited to slight extent, the results from DHA/DHEAS might not be necessarily due to the PPARα activation alone. Thus, we assume that upregulation of PPARα by DHEAS could be a trigger to induce apoptosis or cell death signaling rather than direct stimulation of apoptosis in VSMCs by DHEAS. Although the entire mechanism of DHEAS-induced modulation of VSMC activity still remains unclear, the responsible mechanism for the effect of DHEAS on VSMC is considered to be, at least in part, due to upregulation of p16INK4a and activation of PPARα–involved signaling. Apart from the suppressive effect of DHEAS on VSMC functions in vitro and in vivo which is one of the major mechanistic insights in this study, DHEAS also demonstrated an potent inhibitory effect on inflammation of vessel wall following balloon injury as reported previously.16 Indeed, immunohistochemical analysis exhibited the clearly reduced neutrophil/T-cell infiltration in the DEHAS-treated artery compared with that in the control artery following balloon injury. (supplemental figure V)
Our data suggest that one of the adrenal androgens, DHEAS, may play a role for vascular remodeling after arterial injury promoting the effect of exogenous PPARα ligands e.g. fish oil (DHA) and fibrates ordinarily taken in our daily life and reducing vascular inflammation. The replacement therapy of DHEAS in male human subject may have a beneficial effect for preventing atherosclerosis or restenosis after catheter-based arterial intervention in the clinical setting.
Acknowledgments
This work was supported by a grant from the Osaka Medical Research Foundation for Incurable Disease and in part by NIH grants (HL-53354, HL-60911, HL-63414, HL-63695, HL-66957).
Footnotes
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vermeulen A. Dehydroepiandrosterone sulfate and aging. Ann N Y Acad Sci. 1995;774:121–127. doi: 10.1111/j.1749-6632.1995.tb17376.x. [DOI] [PubMed] [Google Scholar]
- 2.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell LE, Sprecher DL, Borecki IB, Rice T, Laskarzewski PM, Rao DC. Evidence for an association between dehydroepiandrosterone sulfate and nonfatal, premature myocardial infarction in males. Circulation. 1994;89:89–93. doi: 10.1161/01.cir.89.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E, Goodman-Gruen D. The epidemiology of DHEAS and cardiovascular disease. Ann N Y Acad Sci. 1995;774:259–270. doi: 10.1111/j.1749-6632.1995.tb17386.x-i1. [DOI] [PubMed] [Google Scholar]
- 5.Slowinska-Srzednicka J, Zgliczynski S, Ciswicka-Sznajderman M, Srzednicki M, Soszynski P, Biernacka M, Woroszylska M, Ruzyllo W, Sadowski Z. Decreased plasma dehydroepiandrosterone sulfate and dihydrotestosterone concentrations in young men after myocardial infarction. Atherosclerosis. 1989;79:197–203. doi: 10.1016/0021-9150(89)90124-x. [DOI] [PubMed] [Google Scholar]
- 6.Gordon GB, Bush DE, Weisman HF. Reduction of atherosclerosis by administration of dehydroepiandrosterone. A study in the hypercholesterolemic New Zealand white rabbit with aortic intimal injury. J Clin Invest. 1988;82:712–720. doi: 10.1172/JCI113652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eich DM, Nestler JE, Johnson DE, Dworkin GH, Ko D, Wechsler AS, Hess ML. Inhibition of accelerated coronary atherosclerosis with dehydroepiandrosterone in the heterotopic rabbit model of cardiac transplantation. Circulation. 1993;87:261–269. doi: 10.1161/01.cir.87.1.261. [DOI] [PubMed] [Google Scholar]
- 8.Arad Y, Badimon JJ, Badimon L, Hembree WC, Ginsberg HN. Dehydroepiandrosterone feeding prevents aortic fatty streak formation and cholesterol accumulation in cholesterol-fed rabbit. Arteriosclerosis. 1989;9:159–166. doi: 10.1161/01.atv.9.2.159. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Esaki T, Muto E, Kano H, Asai Y, Thakur NK, Sumi D, Jayachandran M, Iguchi A. Dehydroepiandrosterone retards atherosclerosis formation through its conversion to estrogen: the possible role of nitric oxide. Arterioscler Thromb Vasc Biol. 2000;20:782–792. doi: 10.1161/01.atv.20.3.782. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann R, Mintz GS, Dussaillant GR, Popma JJ, Pichard AD, Satler LF, Kent KM, Griffin J, Leon MB. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996;94:1247–1254. doi: 10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- 13.Furutama D, Fukui R, Amakawa M, Ohsawa N. Inhibition of migration and proliferation of vascular smooth muscle cells by dehydroepiandrosterone sulfate. Biochim Biophys Acta. 1998;1406:107–114. doi: 10.1016/s0925-4439(97)00085-9. [DOI] [PubMed] [Google Scholar]
- 14.Peters JM, Zhou YC, Ram PA, Lee SS, Gonzalez FJ, Waxman DJ. Peroxisome proliferator-activated receptor alpha required for gene induction by dehydroepiandrosterone-3 beta-sulfate. Mol Pharmacol. 1996;50:67–74. [PubMed] [Google Scholar]
- 15.Hampl V, Bibova J, Povysilova V, Herget J. Dehydroepiandrosterone sulphate reduces chronic hypoxic pulmonary hypertension in rats. Eur Respir J. 2003;21:862–865. doi: 10.1183/09031936.03.00084503. [DOI] [PubMed] [Google Scholar]
- 16.Altman R, Motton DD, Kota RS, Rutledge JC. Inhibition of vascular inflammation by dehydroepiandrosterone sulfate in human aortic endothelial cells: roles of PPARalpha and NF-kappaB. Vascul Pharmacol. 2008;48:76–84. doi: 10.1016/j.vph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negoro N, Hoshiga M, Seto M, Kohbayashi E, Ii M, Fukui R, Shibata N, Nakakoji T, Nishiguchi F, Sasaki Y, Ishihara T, Ohsawa N. The kinase inhibitor fasudil (HA-1077) reduces intimal hyperplasia through inhibiting migration and enhancing cell loss of vascular smooth muscle cells. Biochem Biophys Res Commun. 1999;262:211–215. doi: 10.1006/bbrc.1999.1129. [DOI] [PubMed] [Google Scholar]
- 18.Ii M, Hoshiga M, Fukui R, Negoro N, Nakakoji T, Nishiguchi F, Kohbayashi E, Ishihara T, Hanafusa T. Beraprost sodium regulates cell cycle in vascular smooth muscle cells through cAMP signaling by preventing down-regulation of p27(Kip1) Cardiovasc Res. 2001;52:500–508. doi: 10.1016/s0008-6363(01)00411-4. [DOI] [PubMed] [Google Scholar]
- 19.Nagai R, Kuro-o M, Babij P, Periasamy M. Identification of two types of smooth muscle myosin heavy chain isoforms by cDNA cloning and immunoblot analysis. The Journal of biological chemistry. 1989;264:9734–9737. [PubMed] [Google Scholar]
- 20.Kuro-o M, Nagai R, Nakahara K, Katoh H, Tsai RC, Tsuchimochi H, Yazaki Y, Ohkubo A, Takaku F. cDNA cloning of a myosin heavy chain isoform in embryonic smooth muscle and its expression during vascular development and in arteriosclerosis. The Journal of biological chemistry. 1991;266:3768–3773. [PubMed] [Google Scholar]
- 21.Ruoff BM, Daniel WL. Comparative biochemistry of mammalian arylsulfatase C and steroid sulfatase. Comp Biochem Physiol B. 1991;98:313–322. doi: 10.1016/0305-0491(91)90184-f. [DOI] [PubMed] [Google Scholar]
- 22.Sakyo K, Ito A, Mori Y. Dehydroepiandrosterone sulfate stimulates collagenase synthesis without affecting the rates of collagen and noncollagen protein syntheses by rabbit uterine cervical fibroblasts. Biol Reprod. 1987;36:277–281. doi: 10.1095/biolreprod36.2.277. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Nakamura T, Takagaki K, Funahashi M, Saito Y, Endo M. Regulation of hyaluronate metabolism by progesterone in cultured fibroblasts from the human uterine cervix. FEBS Lett. 1997;402:223–226. doi: 10.1016/s0014-5793(96)01538-4. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi K, Saga H, Chimori Y, Kimura K, Yamanaka Y, Sobue K. Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase. The Journal of biological chemistry. 1998;273:28860–28867. doi: 10.1074/jbc.273.44.28860. [DOI] [PubMed] [Google Scholar]
- 25.Wiegman PJ, Barry WL, McPherson JA, McNamara CA, Gimple LW, Sanders JM, Bishop GG, Powers ER, Ragosta M, Owens GK, Sarembock IJ. All-trans-retinoic acid limits restenosis after balloon angioplasty in the focally atherosclerotic rabbit: a favorable effect on vessel remodeling. Arterioscler Thromb Vasc Biol. 2000;20:89–95. doi: 10.1161/01.atv.20.1.89. [DOI] [PubMed] [Google Scholar]
- 26.Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- 27.Diep QN, Touyz RM, Schiffrin EL. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension. 2000;36:851–855. doi: 10.1161/01.hyp.36.5.851. [DOI] [PubMed] [Google Scholar]