Abstract
It is well known that hydroxyl radicals are generated by ultrasound in water. This study with an electron spin resonance spin-trapping technique showed that hydroxyl radical generation was positively correlated with ultrasound duration and water temperature. The clear fungicidal action against Trichophyton spp. evident by studying cultured cells and the degradation of cytoplasmic and surface structures observed by transmission and scanning electron microscopy suggest that ultrasound in hot water is effective for sterilization of dermatophyte contamination and could be effective for the treatment of tinea infection.
Keywords: ultrasound, hydroxyl radical, Trichophyton spp., fungicidal activity, electron microscopy
Introduction
Fungal infections in humans are mainly classified according to infection site into two categories, superficial and systemic, and the pathogenic fungi causing superficial infections preferably inhabit keratinized tissues such as stratum corneum of the skin, nail, and hair. The tinea corporis and tinea pedis caused by Trichophyton spp., cutaneous candidiasis caused by Candida spp., and tinea verisicolor caused by Malasezzia furfur are successfully treated by recently developed topical antifungal drugs [1]. In contrast to these superficial fungal infections, tinea unguium or onychomycosis caused by Trichophyton spp. is usually resistant to topical antifungal chemotherapy and is therefore treated with oral drugs such as itraconazole, an azole antifungal drug, and terbinafine, an allylamine antifungal drug [2–6]. Itraconazole capsules, for instance, are given in a pulse-treatment regimen (400 mg/day for 1 week at monthly intervals) that is repeated twice or three times for finger nail onychomycosis and is repeated three or four times for toenail onychomycosis [4–6]. Because some strains of Trichophyton spp., however, are resistant to azole compounds [7] and itraconazole has toxic side effects [8], we want to provide a new tool for the treatment of onychomycosis. Antifungal effects of reactive oxygen species have long been documented [9–13], and hydroxyl radicals are known to damage proteins, lipids, and nucleic acids [14–17]. Since hydroxyl radicals are generated when water is exposed to ultrasound [18, 19], we evaluated the fungicidal effects seen when suspensions of the dermatophytes that are the major cause of onychomycosis are exposed to ultrasound.
Materials and Methods
Reagents
5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) of analytical grade was purchased from Labotec Co., Ltd. (Tokyo, Japan), and all the other reagents used were also of analytical grade.
Fungus preparation
Two clinical isolates of Trichophyton mentagrophytes and three clinical isolates of Trichophyton rubrum were obtained from patients with tinea pedis in Showa University Fujigaoka Hospital (Yokohama, Japan), and for each strain a conidial suspension in sterile physiological saline containing 0.1% (w/v) Tween 80 was prepared from cultures grown on Sabouraud dextrose agar slants at 27°C for 1–4 weeks. Following filtration through sterilized gauze to remove hyphal fragments and pieces of agar, the concentration of each suspension was adjusted to 106 conidia/ml.
ESR analyses of hydroxyl radicals generated by ultrasound in water
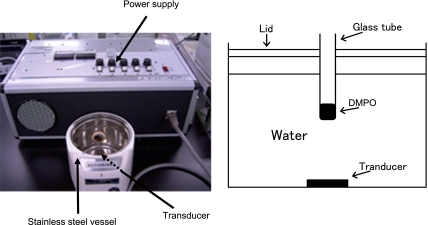
Our device using 1.6-MHz ultrasound to generate hydroxyl radicals is shown schematically in Fig. 1. A glass tube (15 mm in diameter and 85 mm long) containing 1 ml of 89 mM DMPO dissolved in pure water was set into the device and exposed to sonication for 1 min. The reaction mixture obtained after the exposure was immediately transferred to an ESR spectrometry cell, and its ESR spectrum was recorded. The spin concentration of the DMPO-OH (a DMPO and hydroxyl radical adduct) was determined from the double integrals of the DMPO-OH peak by comparing them with those of the peak for the stable nitroxide radical, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl. The measurement conditions for ESR were as follows: field sweep, 330.50 to 340.50 mT; field modulation frequency, 100 kHz; field modulation width, 0.08 mT; amplitude, 10–100; sweep time, 1 min; time constant, 0.03 s; microwave frequency, 9.420 GHz; microwave power, 4 mW.
Fig. 1.
Photograph and schematic illustration of ultrasound device for hydroxyl radical generation.
Measurement of fungicidal activity by ultrasound radiation
One ml of each conidial suspension was exposed to ultrasound radiation and then diluted with phosphate-buffered saline. A small aliquot was inoculated on a Sabouraud dextrose agar plate and cultured at 27°C for 5 to 14 days. Fungicidal activity was assessed by counting the colonies grown on the agar plate in order to determine the number of colony-forming units (CFUs) that had survived the ultrasound treatment.
Scanning electron microscopy (SEM)
A sample of each conidial suspension was smeared on a silicon-coated glass plate and fixed with 2.5% (w/v) glutaraldehyde in 0.2 M cacodylate buffer (pH 7.4) at 4°C overnight. After washing with 0.1 M cacodylate buffer (pH 7.4), the sample specimens were post-fixed with 2% (w/v) OsO4 in 0.2 M cacodylate buffer (pH 7.4) at 4°C for 2 h and then routinely processed for scanning electron microscopic observation. In brief, the post-fixed specimens were dehydrated through an ethanol series, substituted with isoamyl acetate, and dried with liquid CO2 by using a ID-2 critical point dryer (Eiko Ltd., Tokyo, Japan). Each dried specimen was coated with gold by using an SC7640 sputter coater (Quorum Technologies Ltd., Hailsham, UK) and observed by using a Topcon DS701 scanning electron microscope at 10 kV.
Transmission electron microscopy (TEM)
After conidial suspensions of each fungus were washed in physiological saline several times and centrifuged, the resultant pellets were fixed with 2.5% (w/v) glutaraldehyde in 0.2 M cacodylate buffer (pH 7.4) at 4°C overnight. After washing with 0.1 M cacodylate buffer (pH 7.4), the specimens were post-fixed with 2% (w/v) OsO4 in 0.2 M cacodylate buffer (pH 7.4) at 4°C for 2 h, and were routinely processed for transmission electron microscopic observation. In brief, the post-fixed specimens were dehydrated through an ethanol series, substituted with propylene oxide, and embedded in Epon812 so that ultra-thin sections could be prepared. The sections were stained with uranyl acetate and lead citrate and were observed by using a Hitachi H7600 transmission electron microscope (Hitachi High-Technologies Corp., Tokyo, Japan) at 75 kV.
Results and Discussion
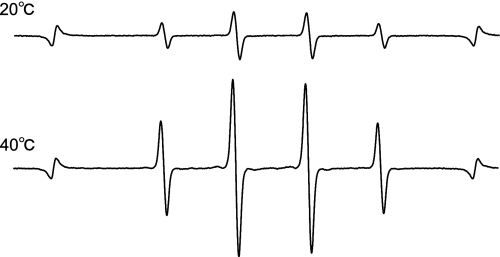
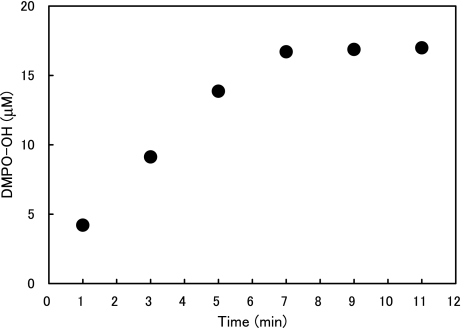
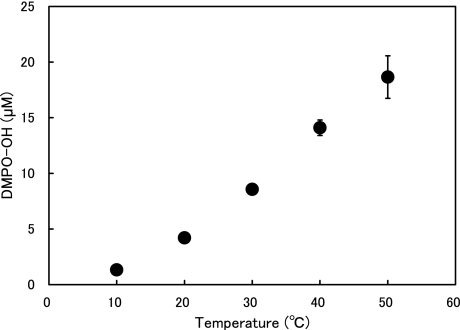
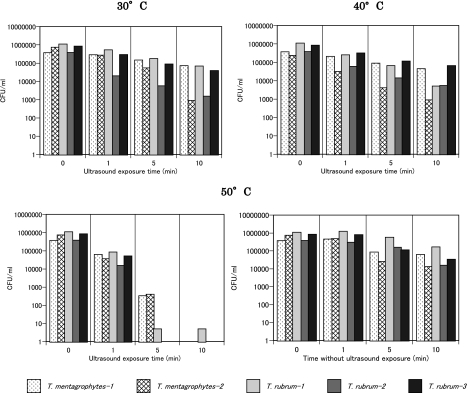
Fig. 2 shows the representative ESR spectra of DMPO-OH generated by ultrasound in water. The hyperfine coupling constants of the ESR signal were aN = aH = 1.49 mT. The effects of water temperature and exposure time on the amount of ·OH generated when water was exposed to ultrasound are summarized in Figs. 3 and 4, where one sees that the DMPO-OH concentration increased with time and with increasing temperature. The effects of water temperature and exposure time on the fungicidal activity of water exposed to ultrasound are shown in Fig. 5, where one sees that the fungicidal effect of ultrasound radiation was enhanced with time and increasing water temperature and that four of the five strains tested did not survive 10 min in 50°C water exposed to ultrasound. One also sees from the bottom-right bar in Fig. 5 that 50°C without ultrasound had almost no fungicidal effect. Since hydroxyl radical generation increased with ultrasound duration and water temperature, the fungicidal action shown in Fig. 5 is likely to be due to attributable to hydroxyl radicals generated by ultrasound in water.
Fig. 2.
Representative ESR spectra of DMPO-OH generated by the ultrasound system at 20°C and 40°C.
Fig. 3.
DMPO-OH concentration vs ultrasound duration.
Fig. 4.
DMPO-OH concentration vs water temperature.
Fig. 5.
Effects of radiation time and water temperature on the fungicidal action of the ultrasound system. The bottom right bar graphs show the effect of 50°C water not exposed to ultrasound.
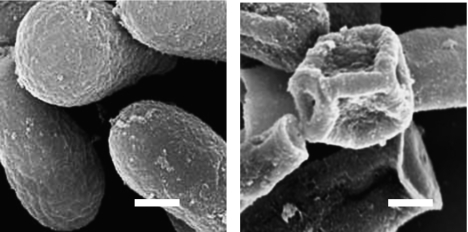
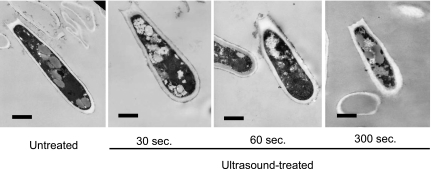
A SEM image of T. mentagrophytes conidia exposed for 5 min to ultrasound in water at 50°C (Fig. 6) shows that the ultrasound caused the conidia to collapse and their surfaces to exfoliate. TEM images of T. mentagrophytes conidia exposed to ultrasound radiation at 50°C for different periods of time (Fig. 7) indicate that the exposure degraded the cytoplasmic structure in ways that are likely to lead to necrosis [20–22]. In the references, the term “necrosis” is used to describe irreversible structural degeneration such as crushed, bent, and flattened cells.
Fig. 6.
SEM images of T. mentagrophytes conidia after 5 min in 50°C water exposed (right) or not exposed (left) to ultrasound. Scale bar: 1 µm.
Fig. 7.
TEM images of T. mentagrophytes conidia after 0 to 5 min in 50°C water exposed to ultrasound. Scale bar: 1 µm.
Ultrasound in water has thus been shown to generate hydroxyl radicals that degrade cytoplasmic and surface structures and thereby lead to necrosis of fungal cells. Since the dermatophytes used in this study are major causes of onychomycosis, ultrasound in hot water is expected to be a useful treatment for onychomycosis. In a future study, to check if ultrasound itself has fungicidal action, we will further examine the effect of specific scavengers of the hydroxyl radical on the fungicidal action of ultrasound in water.
References
- 1.Niwano Y. In: Antifungal Drugs in Fungal Infections. in Handbook of Fungal Biotechnology, Second Edition. Arora K., Bridge P., Bhatnagar D., editors. Marcel Dekker, Inc.; NY, USA: 2003. pp. 453–468. [Google Scholar]
- 2.Goodfield M.J. Short-duration therapy with terbinafine for dermatophyte onychomycosis: a multicenter trial. Br. J. Dermatol. 1992;126 Suppl. 39:33–35. doi: 10.1111/j.1365-2133.1992.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 3.van der Schroeff J.G., Cirkel P.K., Crijns M.B., Van Dijk T.J., Govaert F.J., Groeneweg D.A., Tazelaar D.J., De Wit R.F., Wuite J. A randomized treatment duration-finding study of terbinafine in onychomycosis. Br. J. Dermatol. 1992;126 Suppl. 39:36–39. doi: 10.1111/j.1365-2133.1992.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 4.De Doncker P., Van Lint J., Dockx P., Roseeuw D. Pulse therapy with one-week itraconazole monthly for three or four months in the treatment of onychomycosis. Cutis. 1995;56:180–183. [PubMed] [Google Scholar]
- 5.Warnock D.W. Itraconazole pulse: an overview of current use. Hosp. Med. 1998;59:309–311. [PubMed] [Google Scholar]
- 6.Chen J., Liao W., Wen H., Wu J., Yao Z. A comparison among four regimens of itraconazole treatment in onychomycosis. Mycoses. 1999;42:93–96. doi: 10.1046/j.1439-0507.1999.00404.x. [DOI] [PubMed] [Google Scholar]
- 7.Méndez-Tovar L.J., Manzano-Gayosso P., Velásquez-Hernández V., Millan-Chiu B., Hernández-Hernández F., Mondragón-González R., López-Martínez R. Resistance to azolic compounds in clinical Trichophyton spp. strains. Rev. Iberoam. Micol. 2007;24:320–322. doi: 10.1016/s1130-1406(07)70065-7. (in Spanish) [DOI] [PubMed] [Google Scholar]
- 8.Takahashi N., Kameoka Y., Yamanaka Y., Ubukawa K., Saito K., Fujishima M., Fujishima N., Saito H., Hirokawa M., Scott S.A., Sawada K. Itraconazole oral solution enhanced vincristine neurotoxicity in five patients with malignant lymphoma. Intern. Med. 2008;47:651–653. doi: 10.2169/internalmedicine.47.0701. [DOI] [PubMed] [Google Scholar]
- 9.King D.A., Sheafor M.W., Hurst J.K. Comparative toxicities of putative phagocyte-generated oxidizing radicals toward a bacterium (Escherichia coli) and a yeast (Saccharomyces cerevisiae) Free Radic. Biol. Med. 2006;41:765–774. doi: 10.1016/j.freeradbiomed.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Jupin C., Parant M., Chedid L. Involvement of reactive oxygen metabolites in the candidacidal activity of human neutrophils stimulated by muramyl dipeptide or tumor necrosis factor. Immunobiology. 1989;180:68–79. doi: 10.1016/S0171-2985(89)80031-2. [DOI] [PubMed] [Google Scholar]
- 11.Desai G., Nassar F., Brummer E., Stevens D.A. Killing of Histoplasma capsulatum by macrophage colony stimulating factor-treated human monocyte-derived macrophages: role for reactive oxygen intermediates. J. Med. Microbiol. 1995;43:224–229. doi: 10.1099/00222615-43-3-224. [DOI] [PubMed] [Google Scholar]
- 12.Xie Q., Kawakami K., Kudeken N., Zhang T., Qureshi M.H., Saito A. Different susceptibility of three clinically isolated strains of Cryptococcus neoformans to the fungicidal effects of reactive nitrogen and oxygen intermediates: possible relationships with virulence. Microbiol. Immunol. 1997;41:725–731. doi: 10.1111/j.1348-0421.1997.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 13.Kudeken N., Kawakami K., Saito A. Role of superoxide anion in the fungicidal activity of murine peritoneal exudate macrophages against Penicillium marneffei. Microbiol. Immunol. 1999;43:323–330. doi: 10.1111/j.1348-0421.1999.tb02412.x. [DOI] [PubMed] [Google Scholar]
- 14.Schmidley J.W. Free radicals in central nervous system ischemia. Stroke. 1990;21:1086–1090. doi: 10.1161/01.str.21.7.1086. [DOI] [PubMed] [Google Scholar]
- 15.Farber J.L. Mechanisms of cell injury by activated oxygen species. Environ. Health Perspect. 1994;102 (Suppl 10):17–24. doi: 10.1289/ehp.94102s1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masaki H., Atsumi T., Sakurai H. Detection of hydrogen peroxide and hydroxyl radicals in murine skin fibroblasts under UVB irradiation. Biochem. Biophys. Res. Commun. 1995;206:474–479. doi: 10.1006/bbrc.1995.1067. [DOI] [PubMed] [Google Scholar]
- 17.Ueda J., Takai M., Shimazu Y., Ozawa T. Reactive oxygen species generated from the reaction of copper(II) complexes with biological reductants cause DNA strand scission. Arch. Biochem. Biophys. 1998;357:231–239. doi: 10.1006/abbi.1998.0811. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y., Zhang Z., Yang C. Measurement of hydroxyl radical production in ultrasonic aqueous solutions by a novel chemiluminescence method. Ultrason. Sonochem. 2008;15:665–672. doi: 10.1016/j.ultsonch.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Sato E., Kohno M., Nakashima T., Niwano Y. Ciclopirox olamine directly scavenges hydroxyl radical. Int. J. Dermatol. 2008;47:15–18. doi: 10.1111/j.1365-4632.2007.03359.x. [DOI] [PubMed] [Google Scholar]
- 20.Nimura K., Niwano Y., Ishiduka S., Kato M. Actin gene-targeted RT-PCR could be a useful method for evaluating in vitro fungicidal activity against dermatophytes. J. Int. Med. Res. 2003;31:407–412. doi: 10.1177/147323000303100508. [DOI] [PubMed] [Google Scholar]
- 21.Borgers M., Van de Ven M.A. Degenerative changes in fungi after itraconazole treatment. Rev. Infect. Dis. 1987;9 (Suppl 1):S33–S42. doi: 10.1093/clinids/9.supplement_1.s33. [DOI] [PubMed] [Google Scholar]
- 22.Tameike A., Konomi M., Sato M., Ishijoma S.A., Niwano Y., Osumi M. Structural effects of lanoconazole on the hyphal growth of Trichophyton rubrum. Jpn. J. Med. Mycol. 1996;37:251–261. (in Japanese) [Google Scholar]