Summary
Bluetongue is a vector-borne viral disease of ruminants that is endemic in tropical and subtropical countries. Since 1998 the virus has also appeared in Europe. Partly due to the seriousness of the disease bluetongue virus (BTV), a member of genus Orbivirus within the family Reoviridae, has been a subject of intense molecular study for the last three decades and is now one of the best understood viruses at the molecular and structural levels. BTV is a complex non-enveloped virus with seven structural proteins arranged in two capsids and a genome of ten double-stranded (ds) RNA segments. Shortly after cell entry the outer capsid is lost to release an inner capsid (the core) which synthesises capped mRNAs from each genomic segment, extruding them into the cytoplasm. This requires the efficient co-ordination of a number of enzymes including helicase, polymerase and RNA capping activities. This review will focus on our current understanding of these catalytic proteins as derived from the use of recombinant proteins, combined with functional assays and the in vitro reconstitution of the transcription/replication complex. In some cases, 3D structures have complemented this analysis to reveal the fine structural detail of these proteins. The combined activities of the core enzymes produce infectious transcripts necessary and sufficient to initiate BTV infection. Such infectious transcripts can now be synthesised wholly in vitro and, when introduced into cells by transfection, lead to the recovery of infectious virus. Future studies thus hold the possibility of analysing the consequence of mutation in a replicating virus system.
Background
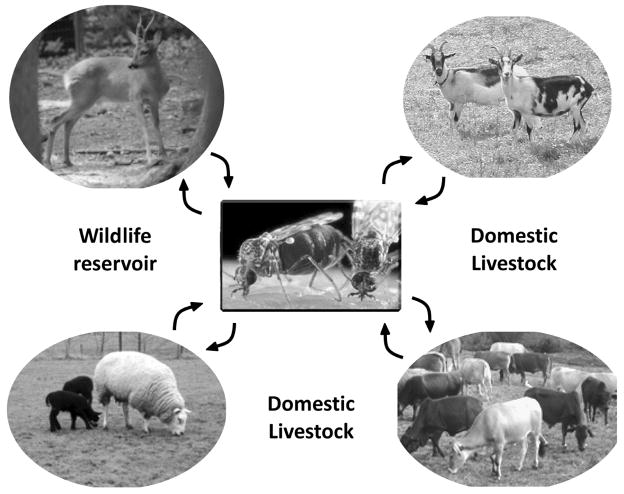
The year 2007 was a landmark for Bluetongue disease in the UK with the first outbreak recorded in East Anglia in September followed by Essex, Cambridgeshire and Kent and, by the end of the year, a number of additional cases in various parts of the country (DEFRA, 2008). For both farmers and the public, the outlook for 2008 is necessarily cautious as this is a newly emerging disease in key livestock, sheep and cattle that had not previously been considered a threat. Bluetongue virus (BTV), which is the causative agent of the disease, has been known for >100 years. Its inexorable spread from its origins in South Africa has led to European colonisation over the last 10 years such that its occurrence in the UK was but a matter a time (EFSA, 2007). BTV causes haemorrhagic disease in ruminants and, as such, represents a major economic threat in many parts of the world. It can infect both wild ruminants and domestic livestock, causing disease in sheep, goats, and cattle with mortality reaching 70% in some breeds of sheep. BTV is endemic in many tropical and sub-tropical countries. The virus is transmitted by several species of biting midges (gnats) in the genus Culicoides (Fig. 1) and, similar to the other arboviruses, the distribution and seasonal activity of these insect vectors determines both the distribution and occurrence of disease in animals (Purse et al., 2005). There are at least 24 serotypes (BTV-1,-2, etc.), with most prevalent in South Africa (Erasmus, 1985). In Europe however, only a handful of the serotypes (BTV-1, -2, -4, -8, -9 and -16) circulate and it is not clear exactly how a particular serotype of the virus arrives or why some seem more successful than others. The lack of a gradual spread of one dominant serotype would suggest that repeated importation of infected animals or the long distance movement of Culicoides vectors are the cause.
Figure 1.
A schematic showing BTV transmission by blood feeding Culicoides from infected to healthy animals that include both wildlife and domestic livestock.
BTV is the type species of the genus Orbivirus within the family Reoviridae. which includes a total of twelve distinct genera (Mertens et al., 2005). Many of these viruses are pathogenic to animals and some also infect or are pathogenic for humans (Orthoreovirus, Rotavirus, Coltivirus and Seadornavirus) (Mertens et al., 2005). Orbiviruses (14 related serogroups, some 140 members) infect animals, plants and insects and are transmitted by arthropods, such as mosquitoes, gnats and ticks. Many of these viruses cause diseases in animals or plants, often with high economic impacts in agriculture and animal health. Although BTV and the related epizootic haemorrhagic disease virus (EHDV) of deer, African horse sickness virus (AHSV), and equine encephalosis virus (EEV) are all transmitted by gnats (Culicoides) and can cause high morbidity and mortality in animals, BTV is the more common throughout the world, often causing serious periodic outbreaks (Erasmus, 1985). As a result of its economic significance, BTV has been the subject of extensive molecular, genetic and structural studies and now represents the best characterised of all the Orbiviruses (Roy, 2007).
Viruses of the Reoviridae, including BTV and other orbiviruses, are characterized primarily by their genome of 10–12 segments of linear, double-stranded RNA (dsRNA). Almost all of these separate segments represent single genes, generating a total of 10–13 viral proteins. The virions are non-lipid-containing icosahedral capsid structures, usually with an outer capsid layer surrounding an inner capsid or core that contains the genome. Shortly after cell entry this outer capsid is removed to release the inner capsid within which the genome remains sequestered from the cellular triggers of innate immunity. Cores must necessarily, therefore, carry all the transcription machinery of the virus, synthesising and extruding multiple capped positive (+ve) sense RNAs from each genomic segment into the host cell cytoplasm. Current models for the transcription of the dsRNA genome are based on the polymerase complex contacting the template RNA and the nascent transcript being directed out of the core particle through a pore on its surface. This requires the efficient co-ordination of some half-a-dozen enzyme activities including helicase, polymerase and RNA capping activity. Considerable advances have been made in recent years in understanding the replicase complexes of these viruses, including BTV. Each of the BTV proteins that form the complex has been expressed as a recombinant protein, purified and used to develop an in vitro assay system for activity. This, in turn, has lead to the detailed mapping of the structure-function relationships among each core component. In some cases, three-dimensional structural studies have complemented these analyses to reveal the fine level of structural detail associated with proteins of the BTV inner capsid, their function alone and in combination. This review will be centred on the molecular dissection of these proteins and will discuss recent data that demonstrate how the combined activities of the core enzymes result in the release of infectious transcripts that are necessary and sufficient to establish viral infection.
Overview of BTV replication
Like the other members of the Reoviridae, BTV virions are non-enveloped, architecturally complex structures composed of multiple layers of proteins. In the case of BTV, there are seven structural proteins (VP1-VP7) organised into an outer capsid and an inner capsid (commonly known as a “core”) containing the ten dsRNA segments of the viral genome. Although the basic features of BTV replication cycle are similar to those of other members of the family, such as reoviruses and rotaviruses, BTV and other orbiviruses multiply in arthropods as well as in vertebrate hosts (Fig. 1), resulting in some differences at a finer level. Also, there are several structural differences in virus particles and protein organisations between these viruses and thus it is conceivable that some stages of BTV replication would be unique.
In mammalian cells, BTV entry proceeds via virus attachment to a receptor on the plasma membrane (Eaton & Hyatt, 1989). Through a combination of biochemical and confocal microscopy studies, together with specific inhibitors and RNA interference, it has recently been shown that BTV enters cells by clathrin-mediated endocytosis and pH-dependent penetration (Forzan et al., 2007).
The outer capsid proteins of BTV, which are non-glycosylated, are responsible for virus entry and penetration and their structural organisations must facilitate these processes. Image processing of virion micrographs obtained from cryo-electron microscopy (cryo-EM) show a well-ordered morphology with an unique icosahedral organisation (Hewat et al., 1992a; 1994; Nason et al., 2004). The icosahedral virion particle has a diameter of ~88 nm and the outer layer is composed of 180 VP2 molecules and 360 VP5 molecules. The 180 VP2 molecules form 60 spike-like, sail-shaped structures while the 360 VP5 molecules are arranged in 120 globular structures and are located more internally than the VP2 spikes. The VP2 spikes extend 3nm beyond the main body of the particle and are responsible for virus attachment to the cell surface and receptor-mediated endocytosis of the virion (Eaton & Hyatt, 1989; Forzan et al., 2007). BTV entry into the cytoplasm requires endosomal acidic pH which allows the globular VP5 protein to permeablise the endosomal membrane via its amino terminal ‘pore- forming’ peptide, analogous to the fusion peptides of envelope viruses (Hassan et al., 2001; Forzan et al., 2004; Forzan et al., 2007). The membrane penetration activity of VP5 was dramatically shown when VP5 was presented appropriately on the cell-surface and induced cell-cell fusion, confirming that it has the capability to destabilize cellular membranes (Forzan et al., 2004). Critically, VP5 only exhibits its membrane-destabilising properties after it has undergone a low pH-triggered activation step, which presumably mimics the endosomal environment encountered during cell entry and possibly triggers a change in the conformation of the protein (Forzan et al., 2007). VP5 lacks the autocatalytic cleavage and N-terminal myristoyl group present in the entry proteins of reoviruses and rotaviruses and does not require proteolytic activation in contrast to some other viral fusion proteins (Espejo et al., 1981; Estes et al., 1981; Smee et al., 1981; Nibert et al., 1991; Colman & Lawrence, 2003). In the case of rotavirus, the penetration of virus into the cells’ cytoplasm (and probably also the uncoating of the virus particle) is dependent upon trypsin activation of viral outer capsid protein VP4. It is noteworthy that the structural organisation of the outer capsid proteins of rotavirus and reovirus are very different from that of BTV (Nason et al., 2004; Zhang et al., 2005; see review by Pesavento et al., 2006), although in all three groups of viruses the outer capsid proteins perform essentially same function, i.e., entry, membrane penetration and release of transcriptionally active inner capsid into the cytoplasm.
Thus, for BTV the current model posits that VP2 makes initial contact with the host cell and triggers receptor mediated endocytosis of the virus particle and then VP5 undergoes a low pH-triggered conformational change that results in the destabilisation of the endosomal membrane (Forzan et al., 2007). It is likely that the change in conformation of VP5 that promotes membrane destabilisation, forming a protein layer with intrinsic outside-in curvature, weakens the contacts between VP5 and the underlying outer layer of the core (Forzan et al., 2004; Forzan et al., 2007). This allows core particles, from which both outer capsid proteins have been lost, to be released into the cytoplasm and initiate genome replication (Van Dijk & Huismans, 1982; Huismans et al., 1987). In addition to being transcriptionally active, cores generated in vitro by proteolytic treatment of purified BTV also retain infectivity for the insect vector and vector-derived cells, indicating that core proteins can also mediate cell attachment and penetration (Mertens et al., 1987).
The core is a multi-enzyme complex composed of two major proteins (VP7 and VP3) and three enzymatically active minor proteins (VP1, VP4 and VP6) in addition to the ten segments of dsRNA genome (~19,000 base pairs in total) (Verwoerd et al., 1970; Verwoerd et al., 1972; Fukusho et al., 1989 and see reviews by Roy et al., 1990a; Roy, 1995). Core enzymes transcribe the ten viral genome segments, as well as cap and methylate full-length mRNA copies of each segment (Van Dijk & Huismans, 1982). The mRNAs are not polyadenylated. In the current model of replication, the mRNA molecules synthesised by the parental cores represent the only transfer of genetic information to the next generation of progeny particles. Transcription occurs inside the viral core and involves the extrusion of ‘capped’ and methylated mRNA species that are subsequently translated into viral proteins in the cytoplasm of an infected cell. The genomic dsRNA segments are never released from the core. Biochemical and EM evidence suggests that all ten genome segments are transcribed simultaneously, similar to the reovirus (Gillies et al., 1971; Huismans & Verwoerd, 1973; Bartlett et al., 1974), although the ten mRNA species are not synthesised at the same rate for BTV (Verwoerd & Huismans, 1972).
Newly produced viral proteins later interact with sequestered viral mRNA species within cytoplasmic viral inclusion bodies (VIBs) to form proviral particles. These proviral particles are believed to be the sites of dsRNA synthesis and the further production of mRNA prior to eventual formation of complete virus particles and extrusion/release from an infected cell (Lecatsas, 1968; Eaton et al., 1990). The VIBs, predominantly consist of the viral coded non-structural protein, NS2, which is synthesized abundantly in the virus-infected cells and is responsible for recruiting both the core proteins and newly synthesised transcripts (Thomas et al., 1990; Kar & Roy, 2003; Lymperopoulos et al., 2003; Modrof et al., 2005; Lymperopoulos et al., 2006). Although the exact mechanism of genome encapsidation is still not clear, current data suggests that VIBs are the genome encapsidation and assembly sites of the cores. However, outer capsid proteins are not recruited by NS2 and the assembly of outer capsid on the core does not take place within the VIBs. The two outer capsid proteins are processed independently of each other and outside of the VIBs (Bhattacharya et al., 2007; Kar et al., 2007). The smallest of the NS proteins (NS3), which is encoded by the smallest RNA segment (S10), is the only glycosylated protein encoded by BTV and is found associated with both VP2 and VP5 (French et al., 1989; Wu et al., 1992; Beaton et al., 2002). Current data suggests that NS3 is involved both in virus maturation and release (Hyatt et al., 1993; Beaton et al., 2002; Wirblich et al., 2006). Unlike NS2 and NS3, much less is known of the role of the largest NS protein, NS1, which is encoded by RNA segment 6 and synthesises large numbers of tubular structures in the infected cells (Huismans & Els, 1979; Urakawa & Roy, 1988). This is a unique feature of BTV and other orbiviruses and neither rotavirus nor reovirus infected cells exhibit such tubular structures. Current data suggest that NS1 is an essential protein and is involved in virus replication and morphogenesis (Owens & Roy, 2004). The assembly, roles of the outer capsid and NS proteins in the virus life cycle will not be discussed further in this review as these have recently been reviewed elsewhere (Roy, 2005; Noad & Roy, 2006). However, the functions assigned to each of the ten proteins are summarised in Table 1.
Table 1.
BTV genome segments, encoded proteins, their locations and functions
| Segment No. | Protein | Location in Virion Particle (VP1-VP7) | Function |
|---|---|---|---|
| 1 | VP1 | Inner Core | RNA polymerase |
| 2 | VP2 | Outer shell (spike) | Receptor binding, virus entry, hemagglutinin, type-specific, neutralization |
| 3 | VP3 | Subcore layer (scaffold) | Forms scaffold for VP7 trimers, interacts with transcription complex and genomic RNA |
| 4 | VP4 | Inner core | Capping enzymes-guanylyltransferase, methyltransferases 1 and 2, RNA 5′ triphosphatase, inorganic pyrophosphatase, NTPase |
| 5 | VP5 | Outer shell (globular) | Virus penetration, fusogenic |
| 6 | NS1 | Nonstructural | Forms tubules, involved in virus replication and trafficking |
| 7 | VP7 | Core surface layer | Forms surface of core, responsible for core entry into insect cells |
| 8 | NS2 | Nonstructural | Phosphorylated, recruits BTV single-stranded RNA (ssRNA), forms cytoplasmic inclusion bodies, site for core assembly |
| 9 | VP6 | Inner core | Viral helicase, adenosine triphosphatase (ATPase), forms hexamers in presence of BTV RNA |
| 10 | NS3 | Nonstructural | Glycoproteins, membrane proteins, interact with host membrane proteins |
| NS3A | Calpactin and Tsg101, aids virus release |
The architecture of BTV core particles that facilitate the synthesis and release of viral transcripts
BTV cores are highly robust structures and can be generated in vitro from purified virus particles by protease treatment (Van Dijk & Huismans, 1980; 1988). This has facilitated three-dimensional structural analyses which have revealed that the icosahedral surface of the core (73 nm in diameter) has a triangulation number of 13 in a left-handed configuration (T=13l) and is solely made up of 260 trimers of VP7 that are attached onto an inner, thin protein shell (59 nm in diameter) (Prasad et al., 1992; Grimes et al., 1997; Grimes et al., 1998). The core possesses channels at both the icosahedral three-fold and the five-fold axes (Fig. 2). The three minor proteins, together with genomic RNA, are enclosed by the inner thin shell which is formed essentially by 120 VP3 molecules arranged in 12 decamers. The structural architecture of this layer is also shared by other members of the family, emphasising the importance of this architecture in capsid assembly for these viruses (see review by Stuart & Grimes, 2006). It is likely that assembly of this layer initiates the assembly of the core. Indeed the expressed VP3 alone, or VP3 and VP7 together can assemble into single shells (although often not very stable) or highly stable double-shelled particles in the absence of the minor proteins and genomic RNA (French & Roy, 1990; Hewat et al., 1992b). The self-assembly capability of VP3 indicates that VP3 amino acid sequences not only determine the fold and overall structure of the protein but also the overall size of the complete capsid. The assembly of VP3 must, therefore, also dictate the organisation of the other structural components of the particle that are attached or interact with it, both internally and externally. This notion was further supported by demonstrating that VP3-VP7 recombinant core-like particles also encapsidate the internal minor proteins following co-expression (Loudon & Roy, 1991; Nason et al., 2004). Reconstructions of recombinant particles consisting of only VP3 and VP7 revealed essentially the same size and architecture as that of the authentic cores, while the particles composed of VP3 and VP7, together with two internal proteins, VP1 and VP4, exhibited an extra flower-shaped density directly beneath the icosahedral 5-fold axes and attached to the underside of the VP3 layer (Fig. 2) (Loudon & Roy, 1991; Nason et al., 2004). Further, when the core crystals were soaked in transcription buffer, the 5-fold pores expanded indicating that during transcription, they act as the exit site for mRNA, while low molecular weight metabolites such as nucleotides use separate sites for entry (Diprose et al., 2001). Thus, it appears that there are selective channels for the entry and exit of substrates and by-products into and out of the core, all facilitated by the expansion of the core (Diprose et al., 2001).
Figure 2. Cryoelectron microscopy reconstruction of BTV core.
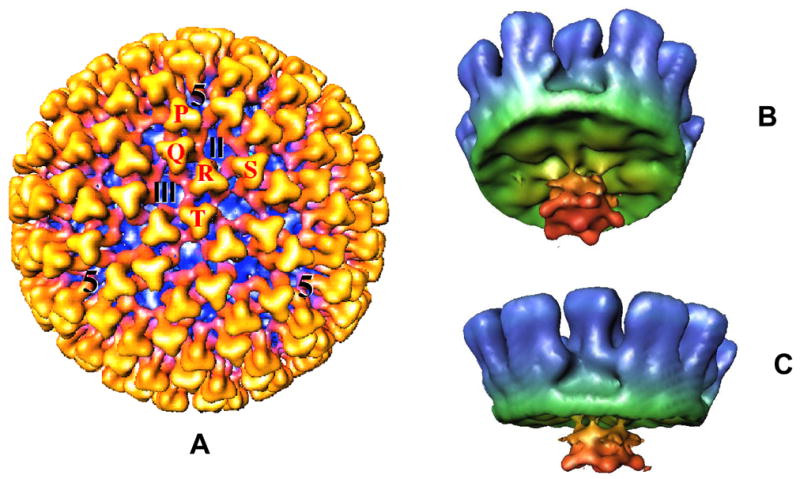
(A): The whole core (700 Å in diameter) viewed along the icosahedral threefold axis showing the VP7 trimers (in yellow). The five quasi-equivalent trimers (P, Q, R, S and T) and the locations of channels are indicated. (B): Cryo-EM structure of a recombinant core-like particle showing the inside view of the CLP reconstruction with VP3 (green), VP7 (blue), VP1 and VP4. A flower-shaped density features (red) attached to the inside surface of VP3 at the 5 fold vertices is made up of VP1 and VP4 (Prasad et al., 1992; Grimes et al., 1997; Nason et al., 2004).
Within the central space of the core crystals, an extra density in layers was visualised and these layers were arranged in a multi-strand helical structure (Grimes et al., 1998; Gouet et al., 1999). The estimated volume of this density was consistent with the molecular mass (~13.1×106 Da) of the BTV genome (19,219 base pairs; (Fukusho et al., 1989), thus implying that these helical layers are the genomic dsRNA segments. The packaging order of these layers appears to be imposed by grooves that form tracks for the RNA on the inside of the VP3 layer but without any specific interactions (Grimes et al., 1998; Gouet et al., 1999). Such an organisation would facilitate the movement of RNA within the core during the transcription activity.
Dissecting the enzymatic function(s) of the core proteins
The fact that the genome of BTV, like other members of the family, remains within the core and only the single-stranded RNA (ssRNA) transcripts are released indicates that replication of the dsRNA genome does not occur via a semiconservative mechanism analogous to that of dsDNA replication. The ssRNA transcripts that are released from the core must also serve as the template on which dsRNA is formed. The conservative mode of replication of dsRNA was demonstrated as early as 1970 for reovirus (Bannerjee & Shatkin, 1970; Silverstein et al., 1970) and was subsequently confirmed by others in the field.
The structural integrity of the core particle appears to be essential for maintaining an efficient transcriptional activity which, in turn, requires the efficient co-ordination of a series of enzyme activities. The BTV core possesses only 3 minor proteins (VP1, VP4, and VP6) which are responsible for synthesising the ‘capped’ and methylated transcripts of each dsRNA segment that are released from the transcribing core into the cytoplasm. In addition to polymerase and capping enzymes, a BTV helicase may also be required to unwind the dsRNA genome prior to the initiation and during the synthesis of mRNA species (Fig. 3). Initial assignment of each catalytic activity was based on the predicted amino acid sequence of each protein (Fukusho et al., 1989; and see review by Roy, 1992). The predicted enzyme activity of each protein was subsequently confirmed by experimental studies. Using individual recombinant proteins and in vitro assay systems, it has been possible to delineate the specific catalytic activities provided by each and to confirm that indeed the three core-associated minor proteins, VP1, VP4 and VP6 are solely responsible for synthesising the ‘capped’ and methylated transcripts of each dsRNA segments. The catalytic activity of each protein established by in vitro systems, together with structural information now available, gives an unambiguous assignment for each protein as discussed below.
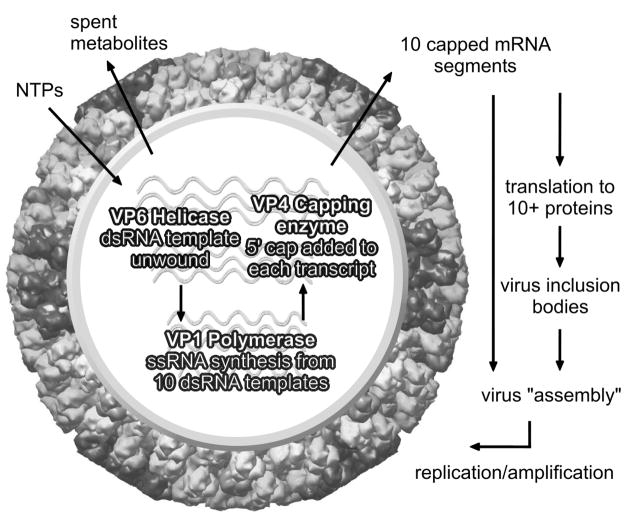
Figure 3. Cartoon of the transcriptionally active core.
Diagram shows the activity of VP1, VP4 and VP6 which form the transcription complex within the core and are responsible for synthesis of ten ‘capped’ mRNA species that are extruded from the core, thereby initiating viral proteins synthesis and subsequent viral replication.
VP6 as a RNA helicase protein
Helicase proteins are responsible for unwinding duplex nucleic acids, DNA, RNA and DNA-RNA hybrids. Two essential features are shared by all helicases; ATP hydrolysis, which releases the energy required for helix destabilization and nucleic acid binding site(s) in order to maintain contact with the nucleic acid that is to be unwound (Lohman et al., 1998). Sequence comparisons of a large number of helicases from different organisms have identified these common functional motifs, including putative sequences for RNA binding, ATP binding/hydrolysis and helicase function (Schmid & Linder, 1992; Kadare & Haenni, 1997; de la Cruz et al., 1999; Dillingham & Kowalczykowski, 2001; Tai et al., 2001). RNA helicases also have a particular signature sequence, the DEAD-box or “DECH-box”, which is responsible for forming the protein-ATP complex in the presence of Mg+2 (de la Cruz et al., 1999; Dillingham & Kowalczykowski, 2001). The smallest BTV core protein, VP6 (35.7 kDa), has a high proportion of hydrophilic and positively charged residues and binds both single-stranded and double-stranded RNA (Roy et al., 1990b). Moreover, in the presence of ATP and magnesium ions, the purified soluble VP6 can bind either blunt-ended RNA duplexes or those with short 5′ or 3′ overhangs and unwind the duplex efficiently in vitro (Fig. 4) (Stauber et al., 1997). These two processes, translocation and strand separation, occur simultaneously during nucleic acid unwinding and use energy from the ATP hydrolysis (Bayliss & Smith, 1996; Paolini et al., 2000; McDougal & Guarino, 2001; White et al., 2001). It is therefore likely that BTV may use VP6 as a helicase, either to unwind the dsRNA ahead of the transcriptase protein or to separate the parental and newly synthesized RNAs following transcription. A number of functional motifs such as ATP binding/ATPase activity, RNA binding and RNA unwinding domains can be recognized in the VP6 protein when it is compared with other available helicases. When these putative functional sites were altered by site-specific mutagenesis, the functional activity of each VP6 mutant derivative was reduced or lost in comparison to the wild-type VP6, emphasising that VP6 is probably the helicase protein of BTV (Kar & Roy, 2003). In particular, substitution at a single residue in the commonly conserved (RxGRxxR) RNA binding motif (RRGRTGR, aa205-211) of BTV VP6 severely affected RNA binding and inhibited RNA unwinding activity. Similarly, changes in the first arginine in the RKGRVGR motif of the vaccinia NPH II protein or HCV NS3 helicase NS3 protein severely reduced RNA binding activity (Gross & Shuman, 1995; Tai et al., 2001). Mutational analysis in HCV NS3 strongly suggests that the first arginine residue forms hydrogen bonds with the key residues involved in RNA binding (Lin & Kim, 1999; Tai et al., 2001). Moreover, in this substitution mutant, ATP hydrolysis was also impaired, indicating that all three functions are interlinked and depend on the same specific sequences within the structure of the protein. The data also suggest that VP6 helicase activity, like all other helicases, is dependent on ATP hydrolysis.
Figure 4. Helicase activity of VP6 and its oligomeric nature.
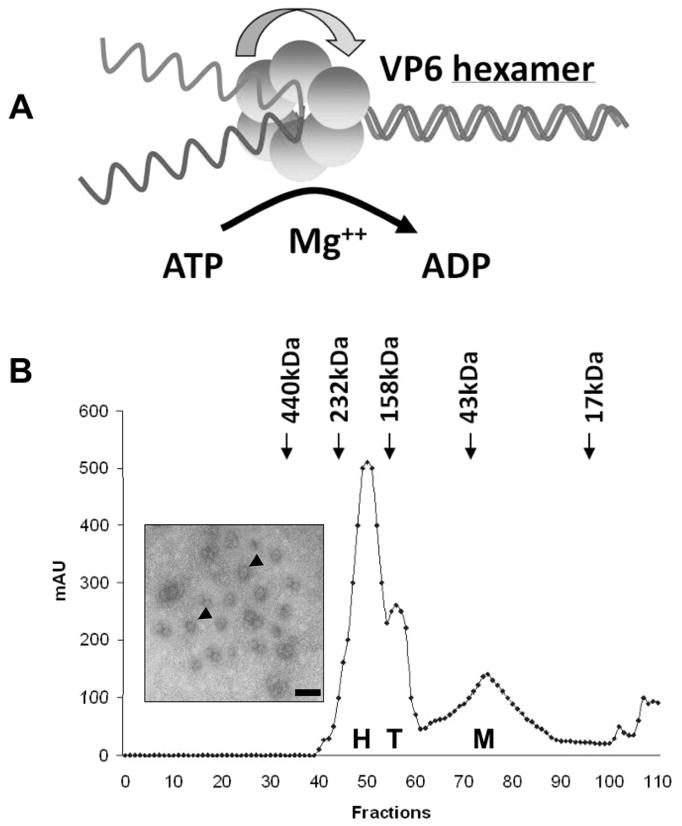
(A). Model of helicase activity of VP6 showing that VP6 binds RNA and unwinds dsRNA to ssRNA product in presence of Mg++ and ATP. (B). Gel filtration analysis of VP6 monomer (M), tetramer (T) and hexamer (H). Inset shows the electron microscopy of ring-like structures formed by VP6 and ss- or dsRNA complexes, stained with 1% uranyl acetate. The scale bar represents 100 Å (Stauber et al., 1997; Kar & Roy, 2003).
Although the exact mechanism of helicase action is still unclear, a number of models have been proposed in which the helicase protein could be active as a monomer, dimer or hexamer (Ahnert & Patel, 1997; Bird et al., 1998; Lohman et al., 1998). The soluble VP6 is a monomer but very rapidly forms stable hexamers, particularly in the presence of ssRNA and dsRNA (Fig. 4). These VP6-RNA complexes form discrete ring-like structure (Fig. 4, Kar et al., 2004). Such ring-like structures are shared by a number of other RNA helicases (Gogol et al., 1991; Sedman & Stenlund, 1998; Fouts et al., 1999). Thus, accumulating data indicates that VP6 is a typical RNA helicase protein with all the functional and structural criteria shared with other viral and cellular helicase proteins.
The largest protein VP1 is the RNA-dependent RNA polymerase
The RNA-dependent RNA polymerase (RdRp) is an essential protein encoded by all RNA viruses that replicates their genome via an RNA intermediate. For BTV, the first indication that the largest BTV protein, VP1 (Mr of 149.5 kDa) is the virus polymerase protein came from sequence comparison with other DNA and RNA polymerases and from a poly(A) polymerase assay system that used a crude extract of insect cells infected with a recombinant baculovirus expressing only BTV VP1 (Fukusho et al., 1989; Urakawa et al., 1989). Subsequent studies have used a purified recombinant protein for an in vitro polymerase assay. These data showed that the soluble BTV VP1 is an active enzyme which exhibits a processive replicase activity in the absence of any other viral proteins and initiates BTV minus-strand synthesis de novo (Boyce et al., 2004). The in vitro reaction conditions for VP1 replicase activity used in these experiments were equivalent to that of the optimal transcriptase activity of intact BTV core particles which indicated that there is no major change in polymerase activity caused by dissociation of VP1 from the rest of the core complex (Van Dijk & Huismans, 1980; Mertens et al., 1984; Van Dijk & Huismans, 1988). These data contrast with those of rotavirus and reovirus. In rotavirus it has been suggested that the assigned polymerase protein (VP1) alone is incapable of performing the polymerase activity in an in vitro assay system, being only functional when it was attached to the inner shell (Chen et al., 1994; Patton et al., 1997; Tortorici et al., 2003). Similarly, it was reported that the assigned polymerase protein of reovirus was incapable of synthesising de novo RNA on its own, although it was shown that a short RNA oligonucleotide could be generated by the protein (Tao et al., 2002). While the BTV VP1 in vitro replicase activity was low, the activity was detectable for at least 24 hours at 37°C. VP1 was able to copy each of the plus-strand RNA segments fully, from the smallest BTV RNA (822 nt) to the largest BTV plus-strand RNA of 3954 nt. Each product was a complete duplex and was RNase I resistant, but RNase III sensitive, dsRNA. Further the minus-strand product was formed by de novo initiation rather than extension of a terminal hairpin-like structure in the template RNA. Surprisingly, given the well-documented template-specific binding of the polymerase of rotavirus (Patton, 1996; Patton et al., 1997; Patton & Chen, 1999; Tortorici et al., 2003), BTV VP1 replicated template RNAs that did not contain the conserved terminal hexanucleotide common to all BTV genome segments. This was also true of templates with termini that bore no resemblance at all to these conserved sequences, although the polymerase did have a preference for templates with a terminal C nucleotide. Interestingly, the reovirus λ3 polymerase, which has been shown to be a poly(C)-dependent poly(G) polymerase (Starnes & Joklik, 1993), is also capable of initiating dsRNA synthesis on a non-viral template in the absence of other viral and cellular proteins (Tao et al., 2002). Thus, BTV and reovirus polymerases show some common characteristics that are quite distinct from rotavirus which has a polymerase that is only active in the presence of the core protein VP2 (Patton et al., 1997; Tortorici et al., 2003). The BTV replicase activity appears to be much more similar to that reported for the dsRNA bacteriophage φ6 polymerase protein P2 with respect to non-specificity. However, P2 does have a preference for templates with the authentic phage-RNA-like dinucleotide at the 3′ end of the template RNA but it will catalyse dsRNA synthesis on any template ssRNA (Makeyev & Bamford, 2000b, 2000a;Makeyev & Bamford, 2001).
The replicase activity of VP1 alone is low, with only a small minority of the potential template molecules being replicated. It is probable that other viral proteins might act normally to modulate the efficient activity of VP1 in the assembling core particle and likely also provide template specificity. Indeed, the evidence from other viral replicase systems would seem to suggest that polymerase activities (e.g., hepatitis C polymerase) are highly regulated in vivo (Piccininni et al., 2002; Shirota et al., 2002).
The confirmation of VP1 as the BTV polymerase protein was further provided by generating a structural model and by reconstitution studies. Since all DNA and RNA polymerases share a similar structure, and as RdRps are more similar to each other than to other polymerases, it was possible to postulate a VP1 3D structure based on several available RdRp crystal structures (Wehrfritz et al., 2007). All RdRp proteins adopt the typical polymerase structure of a right hand, complete with fingers, palm and thumb sub-domains. Co-crystallization of these enzymes with nucleoside triphosphates (NTP, or with oligonucleotides, has mapped substrate binding sites, while the binding of divalent cations, Mg2+ or Mn2+), has been mapped to the catalytic sites, normally characterized by a Gly-Asp-Asp (GDD) motif (Ng et al., 2002; Tao et al., 2002; Choi et al., 2004; Ferrer-Orta et al., 2004). The active site of these polymerases is at the centre of the molecule, in the centre of the palm domain. An additional domain, N-terminal to the fingers, that anchors the tips of the fingers to the thumb is also present in these RdRps (Tao et al., 2002; Choi et al., 2004). Beyond several conserved motifs, there is little primary sequence conservation among the RdRps of the RNA viruses in general, or among those of the dsRNA viruses. Reovirus λ3, which has a total of 1267 amino acids, is a similar size to BTV VP1 (1302 aa residues). The polymerase domain (PD) of λ3 is located in the centre of the molecule (Tao et al., 2002). In addition to PD, unlike the other RdRp, λ3 also possesses a large N- terminal domain (NTD) as well as a C-terminal domain (CTD) (Tao et al., 2002). The NTD of λ3 covers one side of the active site, and anchors the fingertips to the thumb. The CTD of λ3 which covers the catalytic cleft on the other side forms a bracelet structure with two tightly sealed circles. The opening in the centre of the bracelet forms the exit route for the nascent dsRNA (Tao et al., 2002).
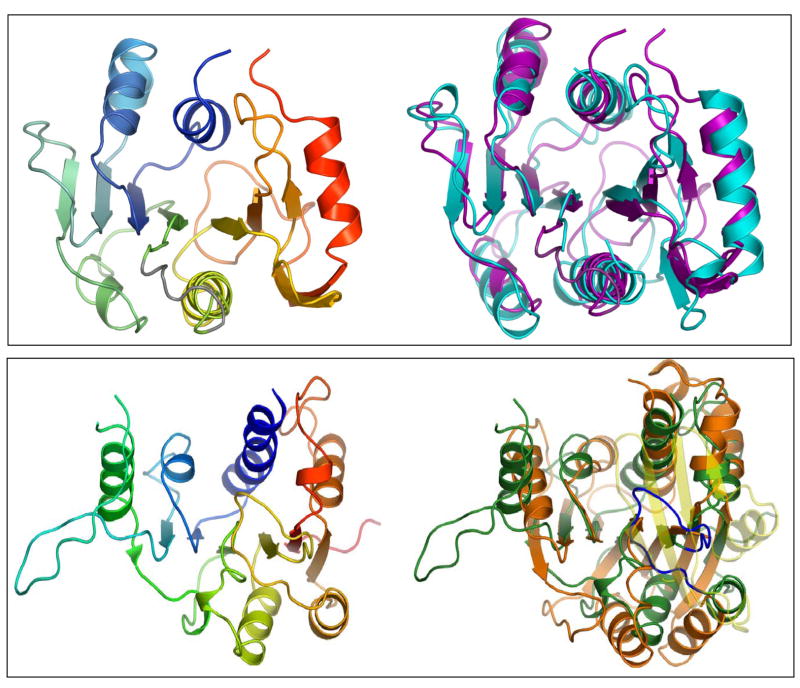
BTV VP1 has a GDD motif at position 763–765 which is surrounded by the other sequence motifs characteristic of polymerase proteins (Roy et al., 1988; Bruenn, 1991; 2003), which suggests that VP1 may have a single, central polymerase domain, similar to reovirus λ3. When submitted to a web-based server, 500 amino acid residues (aa581-880) in the central region of VP1 that include the GDD sequence produced alignments with the RdRps of two positive-sense ssRNA viruses, rabbit haemorrhagic disease virus (RHDV) and poliovirus (PV) (Wehrfritz et al., 2007). To generate a spatially restrained three-dimensional model of the polymerase domain of VP1, the final polymerase sequence alignments were then submitted together to a MODELLER program (Sali et al., 1995). The model shows a typical polymerase structure of VP1 with the canonical structure of a right hand with ‘fingers’, ‘palm’, and ‘thumb’ (Fig. 5). The ‘fingers’ subdomain has three α helices and four β strands in contrast other RdRps which have eight α helices and five or more β strands (Hansen et al., 1997; Ng et al., 2002; Choi et al., 2004; Ferrer-Orta et al., 2004; Appleby et al., 2005).
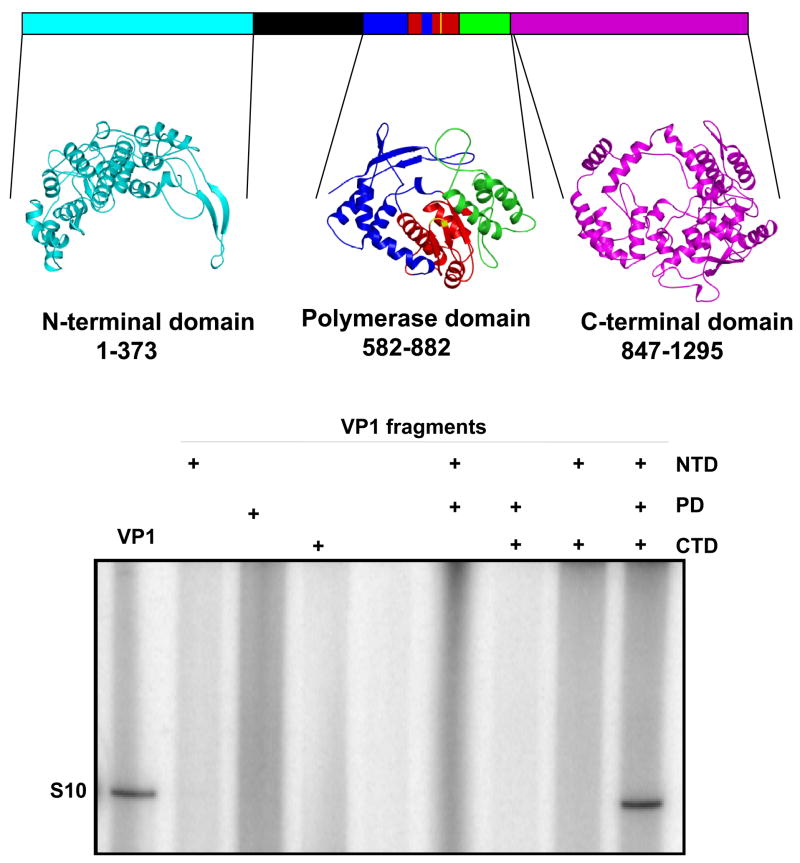
Figure 5. 3D model structure of VP1 and reconstitution of polymerase activity from three VP1 fragments generated using the model.
Upper panel: A structural model of BTV VP1 is generated based on other RNA dependent RNA polymerase structures, showing the PD domain as well as two other additional domains, NTD and CTD that were generated based on reovirus polymerase structure (Tao et al., 2002). The VP1 NTD modelled from amino acid residues 1–373 is shown in cyan. The PD model covers amino acid residues 581–880. Within the PD, the ‘fingers’ subdomain is blue, the ‘palm’ subdomain is red and the ‘thumb’ subdomain is green. The CTD modelled from amino acid residues 847 to 1295 is in magenta. The CTD model overlaps with the ‘thumb’ subdomain of the PD model. A region of VP1 that was not modelled is shown in black. Lower panel: Each of the three (NTD, PD and CTD) VP1 fragments was expressed, purified and tested for replicase activity. Positive controls for the replicase assay were purified VP1 (lane 1) which was at a similar concentration and purity to the fragments. The “+” indicates inclusion of each of the VP1 domains in the lane (Wehrfritz et al., 2007).
The ‘palm’ subdomain predicted for VP1 is composed of a four-stranded antiparallel β sheet flanked by three α helices. This is anarrangement universally found in polymerases. The architecture of the ‘palm’ region is the most highly conserved structure of all known polymerases and many of its features are shared across all families of RNA and DNA polymerases including the VP1 model (O’Reilly & Kao, 1998). The aspartate residues in motif C, which are predicted to be responsible for divalent cation co-ordination, are at positions 764 and 765 of VP1. Two additional aspartate residues believed to be involved in either metal ion coordination or the binding of NTPs are positioned in Motif A at residues 669 and 674 in the VP1 model (Appleby et al., 2005; Wehrfritz et al., 2007)
The ‘thumb’ subdomain of modelled VP1 has three α-helices linked by loops. The ‘thumb’ subdomain of RdRps generally have four or more α helices that are preceded by a short β strand that is located between the ‘palm’ and the ‘thumb’ subdomains (Ng et al., 2002; Tao et al., 2002). In VP1, there is a gap in the polymerase domain alignment which indicates that this β-strand is missing in VP1. The ‘thumb’ of reovirus λ3 polymerase also has only three helices.
Reovirus λ3 is the only RdRp from the Reoviridae for which the structure has been solved. The initial search of the SAM T-02 server database, using the central portion of BTV VP1 did not identify the equivalent regions of reovirus λ3 or bacteriophage φ6 P2, the structures of which are known (Butcher et al., 2001; Tao et al., 2002). However, a subsequent search of the FUGUE server database (Shi et al., 2001) using the full-length VP1 recovered an alignment spanning the entire length of the VP1 protein with the sequence of reovirus λ3, suggesting that the polymerase domain of VP1 could also be modelled on λ3. The modelled regions that were obtained from this alignment showed a polymerase-type structure similar to the model already obtained and similar to the polymerase domain of λ3. A comparison of the root mean square deviation (RMSD) value between the complete polymerase domain model derived from PV and RHDV and the polymerase domain of reovirus λ3 indicated a 2.0 Å deviation (Wehrfritz et al., 2007).
The λ3 structure was also used to model the amino-terminal and carboxy-terminal regions of BTV VP1. The models of both regions exhibited a high degree of structural similarity with these two regions of the λ3 structure (Fig. 5). The VP1 NTD and CTD of VP1 gave RMSD values of 0.9 Å and 0.72 Å, respectively, when compared to the corresponding regions of reovirus λ3. The NTD model of BTV VP1 showed a crescent-shaped, α β protein which was predicted to fit over the PD model anchoring the fingertips to the thumb in a similar fashion to that of λ3 (Tao et al., 2002). However, unlike λ3, in which a cap recognition site has been located in the NTD, no obvious cap recognition residues in VP1 were detected from the alignment used.
The modelled CTD of VP1 has a bracelet structure like that of λ3. In λ3, this bracelet structure forms a pore through which the newly formed genomic dsRNA exits the polymerase. This VP1 domain has putative 20 α helices and 6 β strands, similar to the C-terminal region of λ3, indicating a very close structural similarity in this region of the molecule, and that the CTD of BTV VP1 also must form an exit pore for the nascent dsRNA (Fig. 5). One region in VP1 was impossible to model (Fig 5) and this unmodelled region may be a binding site for either of the two other enzymatic proteins, VP4 or VP6.
To obtain biological evidence that the GDD motif located within the centre of the palm domain is essential for catalytic activity of VP1, this motif was mutated (DD764–765AA) in a recombinant VP1 protein. When tested in vitro, the recombinant mutant protein showed complete loss of catalytic activity, emphasising that the PD model is likely to be correct (Wehrfritz et al., 2007). Further, to verify the model structure biologically, three constructs were designed to express each of the three domains, PD, NTD and CTD, separately in an E. coli expression system. Each expressed fragment was then purified in soluble form and tested for its role in NTP-binding and polymerase activity. Neither the NTD nor the CTD showed any NTP-binding or RdRp activity. Only the PD alone showed efficient NTP binding activity although, it had no RdRp activity. Similarly, when the PD fragment was mixed either with NTD or with CTD, again no RdRp activity was achieved. In contrast, when soluble PD fragment was mixed together with the purified NTD and CTD fragments in vitro, the RdRp activity was reconstituted (Fig. 5) (Wehrfritz et al., 2007). This suggested that, although PD possesses the catalytic activity, the other two domains are needed to stabilise the protein further emphasising that the structure-function relationship of VP1 is analogous to the reovirus λ3 for which the structure has shown that the PD requires the other two domains to stabilise the protein. BTV VP1 is the first polymerase protein to be dissected into three component parts from which a fully functional activity could be reconstituted.
The second largest minor protein VP4 is the mRNA capping enzyme
A fundamental feature of eukaryotic mRNAs is that they are modified co-transcriptionally by addition of a ‘cap’ at their 5′ end. The ‘cap’ is essentially a methyl-guanosine connected via a 5′-5′triphosphate linkage to the first nucleoside of the transcript (Furuichi et al., 1975; Shatkin & Both, 1976). The ‘cap’ structure stabilizes the newly synthesised mRNAs and allows efficient translation initiation of mRNAs (Rottman et al., 1974; Furuichi et al., 1975; Urushibara et al., 1975; Wei et al., 1975; Shatkin & Both, 1976; Parker & Song, 2004). Many eukaryotic viruses including members of the Reoviridae also ‘cap’ the 5′ termini of the RNA transcripts they synthesise. While many viruses use the cellular capping machinery, BTV and reoviruses encode their own capping enzymes. Since the newly synthesised transcripts of these viruses are readily capped prior to their release from the cores, the catalytic activities required for ‘cap’ formation must be provided by one or more proteins within the core. Current models for the transcription of the dsRNA genome of members of the Reoviridae are based on the polymerase complex contacting the template RNA and the nascent transcript being directed out of the core particle through a pore on its surface. In the case of reovirus, this pore is associated with a turret-like structure on the surface of the core that is formed from pentamers of a structural protein which possesses capping activity (Zhang et al., 2003). For rotavirus and BTV, where there are no turret structures present, the capping activity is associated with minor structural components that are located within the inner capsids (Le Blois et al., 1992).
Formation of the cap structure requires at least three key enzymatic activities: (1) an RNA triphosphatase (RTase) that hydrolyses the 5′-triphosphate terminus of the mRNA to a diphosphate; (2) a guanylyltransferase (GTase) that caps the diphosphate terminus with GMP via a 5′-5′ triphosphate linkage, and (3) a guanine-N7-Methyltransferase (N7MTase) that adds a methyl group to the N7 position of the blocking guanosine. For BTV and reovirus transcripts, an additional nucleoside-2′-O-methyltransferase (2′OMTase) is also required. This enzyme is responsible to methylate the 2′-hydroxyl group of the ribose of the first nucleotide (namely type 1 cap). For BTV, based on the predicted amino acid sequence, the second largest minor protein VP4 (76.4 kDa), was predicted to possess some of these catalytic activities. Through the use of highly purified VP4 and recombinant CLPs containing VP4, it was shown that VP4 possesses an RNA 5′triphosphatase activity and can covalently bind GMP via a phosphoamide linkage as well as catalyse a GTP-PPi exchange reaction, both characteristic features of guanylyltransferase enzymes (Martinez Costas et al., 1998; Ramadevi et al., 1998b). Further direct evidence of VP4 capping activity was obtained by demonstrating in vitro transfer of GMP to the 5′ end of in vitro synthesized BTV ssRNA transcripts to form a cap structure. Moreover, VP4 was able to catalyze the conversion of unmethylated GpppG or in vitro-produced uncapped BTV RNA transcripts to a full cap structure, m7GpppGm, in the presence of S-adenosyl-L-methionine. Analysis of the methylated products of the reaction by HPLC identified both methyltransferase type 1 and type 2 activities associated with VP4, demonstrating that the complete BTV capping reaction is associated with this one protein (Ramadevi et al., 1998b). Thus, VP4 alone is responsible for the complete ‘cap’ structure at the 5′ ends of BTV transcripts. Cellular methyltransferase proteins typically appear to encode only a single activity (Reddy et al., 1992), whereas a number of viral methyltransferases, such as that encoded by vaccinia virus, have an additional enzymatic activity such as GTase (Martin et al., 1975; Martin & Moss, 1976). The nsP1 proteins of Semliki Forest virus and Sindbis virus (both positive-strand RNA viruses) also encode both methyltransferase and GTase activities (Mi et al., 1989; Laakkonen et al., 1994). In vaccinia virus, RTase, GTase and N7MTase are components of a capping enzyme complex containing two subunits of 95 kDa and 31 kDa (Venkatesan et al., 1980). However, 2′OMTase activity is mediated by an additional protein VP39 (Barbosa et al., 2002). In contrast to these viruses, BTV VP4 maximizes its coding capacity by catalyzing all of the capping and methylation steps necessary to form the complete type 1 cap structure. The protein also possesses an additional catalytic activity, an inorganic pyrophosphastase activity, which may aid the transcription activity within the virus by removing inorganic pyrophosphate which is an inhibitor of the polymerase reaction (Martinez Costas et al., 1998). BTV VP4 is thus the only capping enzyme in the family for which RTase, GTase and both MTase activities have all been formally demonstrated. This enzyme is unique in combining four capping enzyme activities into a single protein.
Recently, the atomic structure of the protein has revealed how a single protein orchestrates all of these activities (Sutton et al., 2007). To date, almost all structural studies of enzymes associated with cap formation have involved proteins with only one of the activities needed for cap formation. The possible exception to this is reovirus for which a crystal structure of the core is available. In the 3.6 Å resolution structure of the orthoreovirus core, two methyltransferase domains were identified in the λ2 protein (Reinisch et al., 2000; Bujnicki & Rychlewski, 2001). However, as discussed above, the pentameric turret structures that form the orthoreovirus capping complex are missing in the BTV core.
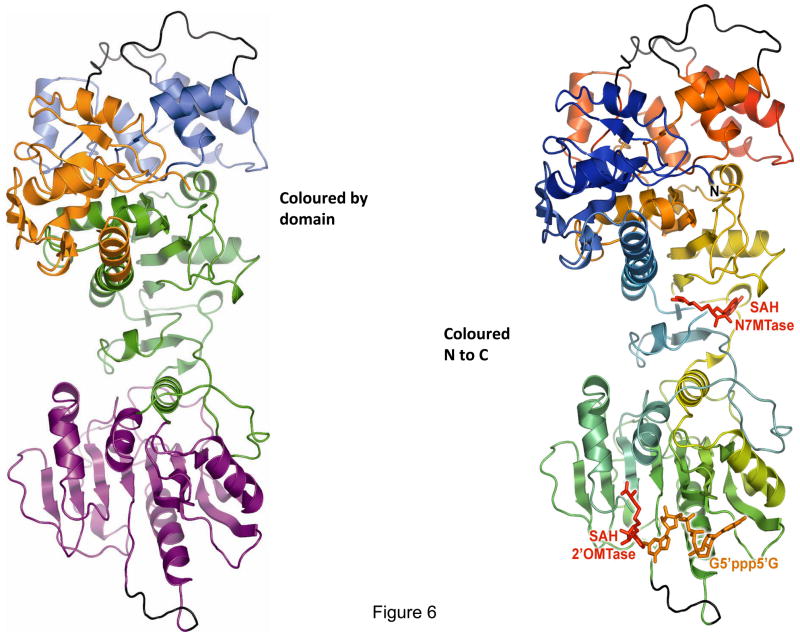
The 2.5 Å resolution crystal structure of recombinant BTV VP4 has revealed how VP4 achieves a series of catalytic activities in the absence of any other core proteins (Sutton et al., 2007). Surprisingly, there is no structural evidence that the capping machinery of BTV and orthoreovirus share a single common ancestor, which may have implications for the evolution of the family Reoviridae. The atomic structure reveals an elongated molecule with four discrete domains that are arranged in linear fashion (Fig. 6). The GTase and possibly the RTase active sites are located as a discrete domain in the C-terminal 135 residues and form a compact stack of six α-helices, while the N7MTase domain (underneath the GTase domain) and the 2′OMTase domain (aa155 to aa377) are located at the centre of the polypeptide. The N7MTase domain is split into two sections (residues 110–154 and 378–509) in between which the 2′OMTase is inserted. Interestingly, an additional domain was identified in the first 108 amino acids of the protein, a kinase-like (KL) domain with architecture similar to other KL folds. KL domains are named after guanylate kinase because they are structurally similar to this enzyme but lack any catalytic activity. However, they have been shown to participate in protein-protein interactions. The KL domain of VP4 may be the site where the VP1 interacts.
Figure 6. Cartoon representation of the X-ray structure of the VP4 molecule.
Ribbon diagram of the VP4 structure showing the four discrete domains (domain nomenclature defined in text) that are arranged in a linear fashion (left). Each domain is coloured as follows: GTase domain (also possibly RTase active site), in red located in the C terminal 135 residues; N7MTase domain (underneath the GTase domain), in orange and 2′OMTase domain, in green are located at the centre of the polypeptide; KL domain (blue) is located in the first 108 residues of the molecule. On the right is the ribbon diagram of the VP4 structure coloured from blue at the N terminus to red at the C terminus, poorly ordered loops are represented in grey. The cartoon in left also shows the ligand (AdoHcy, SAH and G5′ppp5′G) binding sites (Sutton et al., 2007).
There are extensive interactions between some domains in VP4 and a crystallographic 2-fold axis forms a potential dimmer, interface surface of which is contributed by amino acids within the N-terminal 114 and C-terminal 230 residues, in agreement with prior biochemical evidence (Ramadevi et al., 1998a). The GTase and N7MTase domains are closely associated, while the KL domain is reasonably strongly associated with both of these domains. In contrast, the 2′OMT domain is more isolated and only flexibly attached to the remainder of the molecule. But it is structurally similar to the catalytic domains of class I AdoMet-dependent methyltransferases. Superimpositions of the VP4 2′OMT domain on other capping structures show most similarity with the vaccinia virus VP39 structure (Fig. 7), including the signature ‘KDKE’ active site (Bujnicki & Rychlewski, 2001; Egloff et al., 2002), the only RNA 2′OMTase structure to be reported with a bound RNA substrate and cofactor (Hodel et al., 1998). Many of the residues identified as AdoMet/AdoHcy ligands in vaccinia virus VP39 are structurally conserved in BTV VP4 and all other orbivirus VP4 molecules. Further confirmation of the identification of the domains was obtained from analysis of various substrates (GTP, 7mGDP, 5′GpppG5′) and product complexes. Interestingly, both the cap and AdoHcy moieties bound in a very similar fashion to that observed for vaccinia virus VP39, indicating the high conservative nature of the overall structure of this enzyme (Li et al., 2004). Indeed, the 2′OMTase domain appears to be relatively well conserved across many organisms. Unlike the RNA 2′OMTase, very few RNA N7MTases have been determined structurally and the chemistry of the guanine-N7 methylation is distinct from 2′OMTase. Assignment of the N7MTase was based on similarity to the one other N7MT structure (Ecm1) currently in the database and the putative reovirusλ2 N7MT. The overall structure of this domain of VP4 is most similar to Ecm1 of protozoan parasite Encephalitozoon cuniculi mRNA cap (guanine-N7) methyltransferase (Fig. 7), a structure of which is available (Fabrega et al., 2004). Modelling of the Ecm1 cap ligand onto the VP4 structure by superimposition of the protein chains suggests that a group of conserved residues form a pocket for the cap G0 analogous to that observed in Ecm1 confirming further that this is the N7MTase active site of VP4.
Figure 7. Comparisons of VP4 domains with available structures show conservative nature of functional domains.
Upper panel: 2′OMTase VP4 (right) and superimposition (left) of VP4 (purple) and vaccinia virus VP39 (cyan). Lower panel: N7MTase domain of VP4 (right) and superimposition (left) of the N7MTase domain of VP4 with ecm1 (Sutton et al., 2007).
RNA GTases are also varied in structure. The RNA GTases of Chlorella virus (Hakansson et al., 1997) and Candida albicans (Fabrega et al., 2004) show similarity to DNA and RNA ligases and consist of an N-terminal nucleotidyl transferase domain. The structure of the GTase domain of orthoreovirus (Reinisch et al., 2000) is quite different, suggesting that there is more than one family of such enzymes, utilising different structural motifs to execute a common reaction pathway. None of these structures could be identified in the GT domain of VP4 and no similar structures could be detected in the protein data bank. This domain is most likely responsible for the remaining two activities of VP4, RNA-triphosphatase (RTPase) and/or guanylyltransferase (GTase) activity. Indeed, by radiolabeled GTP binding assay followed by analysis of trypsin digested peptides, it has been shown that these two catalytic activities are associated with this domain (Sutton et al., 2007).
The overall layout of active sites in a largely linear fashion along the molecule and the nature of the molecular surface between active sites are consistent with substrate channelling. The efficiency of the process must be optimised by a tight protein-protein interaction with the polymerase enzyme such that the emerging chain is capped almost immediately. Attachment of the KL domain to the polymerase would facilitate this since it is closest to the GT domain that is likely to perform the first two capping reactions on the emerging transcript. However, this possibility will only be confirmed by co-crystallizing the VP1-VP4 complex which is currently under investigation. Thus the combinations of molecular and structural studies have revealed how a single protein can achieve all the catalytic activities required to form the cap 1 structure at the 5′terminus of a de novo BTV RNA transcript.
In summary, each of the three minor proteins of BTV core has the ability to function on its own and together they constitute a molecular motor that can unwind RNAs, synthesise ssRNAs of both polarities and modify the 5′ termini of the newly synthesised mRNA molecules. Much less is known about the in vivo RNA replication mechanisms of BTV. It is believed that, like other members of the family, the packaged plus-strand RNA serves as a template for synthesis of a minus-strand, and, once the minus-strand is synthesized, the dsRNA remains within the nascent progeny particle. As discussed, VP1 acts as the replicase enzyme but the roles of other proteins in minus-strand synthesis remain undefined.
Due to the unique characteristics of BTV VP4 (combining all four capping activities in a single protein), the availability of the atomic structure of this protein represents an important opportunity to completely understand the molecular basis of an mRNA capping mechanism.
BTV transcripts alone in the absence of any proteins can generate infectious virus
The three proteins within the core particles of BTV are responsible for the synthesis and release of capped ssRNA transcripts into the cytoplasm of infected cells, using the genomic dsRNA segments as templates. However, apart from the production of viral ssRNA transcripts, the core particle itself has no other known role in infection. The extruded transcripts act as both mRNAs for the synthesis of the viral proteins and as templates for the synthesis of new dsRNA genome segments. In theory, both these roles would be satisfied if the viral ssRNA was delivered to the host cell cytoplasm by a route other than transcription from an infectious viral core. This notion has prompted an investigation of the possibility that BTV ssRNA could be infectious without the use of any helper virus. Initially viral ssRNA was synthesised in vitro from BTV cores that were purified from virions by chymotripsin treatment and after the transcription reactions, the active cores were then completely removed from the viral ssRNA transcripts. Transfection of these in vitro synthesized ssRNA transcripts into mammalian cells not only initiated all the viral protein synthesis but also led to the recovery of infectious virus (Boyce & Roy, 2007). The ability to recover infectious BTV wholly from ssRNA also suggested a means for establishing a helper virus-independent reverse genetics system for BTV. This was assessed by further extending the manipulation of BTV genes by the addition of one or more plasmid-derived T7 transcripts. Initially, as a proof of concept, it was demonstrated that cells transfected with a mixture of viral mRNAs from two serotypes of BTV could generate reassortant progeny genomes that contain genome segments derived from both parental sets of mRNA (Fig. 8). Further, in vitro synthesized T7 transcripts (either wild-type or mutant variants) derived from cDNA clones were introduced into the genome of BTV using the same system (Boyce et al., 2008). The ability to recover specific mutations in the genome of BTV for the first time not only provides a novel tool for the molecular dissection of BTV and related orbiviruses, but also the opportunity to develop specifically attenuated vaccines to these viruses. More recently, recovery of infectious virus has been shown from transcripts that were generated entirely from ten cDNA clones of the complete genome of BTV serotype (Boyce et al., 2008). The recovered infectious virus showed no difference from either the native virus or the infectious virus recovered from in vitro core-derived transcripts.
Figure 8. Recovery of infectious BTV virions from in vitro synthesized BTV RNA.
10 transcripts were synthesized from BTV core in vitro or generated from cDNA clones. Both type of transcripts produced viral plaques upon transfection of BHK cells (Boyce & Roy, 2007; Boyce et al., 2008).
Alternative reverse genetics strategies have been used successfully for other genera in the Reoviridae (Roner & Joklik, 2001; Komoto et al., 2006; Kobayashi et al., 2007). The first reverse genetics system was a helper virus system for the mammalian orthoreoviruses (Roner & Joklik, 2001). This approach combined reovirus infection of permissive cells and transfection with viral dsRNA, viral mRNA, a T7 transcript, and in vitro translated viral mRNA. Another helper virus approach has allowed the replacement of a rotavirus outer capsid protein with the corresponding protein from another serotype (Komoto et al., 2006). The expression of the introduced genome segment was driven in vivo by the recombinant T7 vaccinia virus system, and selective pressure against the equivalent helper virus protein was provided by the use of antibody selection. Most recently, mammalian orthoreovirus has been recovered using a plasmid-based system similar to the T7 driven systems first used with negative strand viruses (Kobayashi et al., 2007) and, in this case, expression of all ten genome segments was driven in vivo by a recombinant T7 vaccinia virus system.
Successful reverse genetics strategies to date all have several notable features in common: 1) the genome segments derived from cDNA clones are provided as message-sense transcripts in the transfected cell, 2) the cDNA-derived transcripts have the same 5′ end and 3′ end sequences as the corresponding viral transcript (5′ ends are generated through the use of a T7 promoter with the appropriate sequence, and 3′ ends are generated through the use of the hepatitis delta ribozyme in vivo or a restriction enzyme site in vitro), and 3) like authentic viral transcripts, the cDNA-derived transcripts are capped, either in vitro with a cap analogue or in vivo through the cross-capping activity associated with the vaccinia T7 RNA polymerase recombinant (Fuerst et al., 1989). As has been amply demonstrated for other viruses, a reverse genetics system for BTV should contribute to the further understanding of the virus in several research areas. The molecular dissection of BTV protein function to date has mainly been based on recombinant proteins. The ability to introduce specific mutations into the genes of BTV will further our understanding of the functions of these viral proteins in replicating virus and allow the corroboration of the enzymatic or structural functions already assigned. The cis-acting RNA sequences that control the replication, packaging, and expression of orbivirus genomes remain unmapped, and are poorly understood. Reverse genetics will allow the mapping of these regulatory sequences and an investigation of their functions. The replacement of outer capsid proteins can be used to generate vaccine strains with different serotypes based on a common genetic background and it may be possible to identify the determinants of pathogenicity of BTV and related orbiviruses such that strains with varying levels of attenuation could be generated.
Future perspectives
As a result of extensive molecular and structural studies BTV represents an important model system for the study of other viruses. In the infected cells the core of the virus particle is transcritionally active. Its activity marks the beginning of the virus infection cycle and an understanding of its function is therefore fundamental to the biology of virus infection. The structure of the core of dsRNA viruses within the family Reoviridae has revealed a functional molecular machine dedicated to the efficient transcription of RNA upon cell entry, so initiating the infectious cycle. Significant progress has been made in understanding the function of each of the proteins of the BTV core. The present level of understanding has required a merging of structural and molecular studies to map the protein-protein interactions within the core with biochemistry of individual reactions within the protein microenvironment, and represents a novel and holistic approach to uncovering the action of the virion core. It is still the case, however, that the described activity of each individual core protein is incomplete and that a complete understanding of how the core proteins act in concert is lacking. It is becoming increasingly clear that further understanding of the molecular mechanism of these enzymes requires a more thorough understanding of their structure and functional relationship as a complex. Several significant questions still remain, however, regarding the mechanism of the enzymatic reactions that are catalysed by each protein, the assembly of the proteins in the transcriptase complex, how substrates pass from one component to the next, and, in the case of the helicase protein VP6, the precise role of the protein in the transcription process.
Current understanding is that the polymerase complex contacts the template RNA and the dsRNA is used to produce transcripts. How does the polymerase make a copy-choice between the plus- and minus-strand RNAs? The only physical feature that differs between these strands as they are found in the viral genomic RNA is that only the plus-strand RNA has a cap structure. Does VP1 also possesses a cap-binding domain, and is it the recognition of the cap structure juxtaposed with the 3′ end of the viral minus-strand that positions the start of the minus-strand for transcription activity? The purified VP1, VP4 and VP6 have RdRp, capping and helicase activities respectively in vitro, but what is the precise order of contacts between proteins that allow formation of this complex and how does this relate to efficient enzyme function and processivity as a whole?
In addition, the ability to introduce specific mutations into BTV genes, particularly in the polymerase or helicase genes in replicating virus, will allow the corroboration of the enzymatic or structural functions already assigned. Such studies will also reveal if mutations in polymerase or helicase proteins have any significance in virus replication and pathogenesis in the variety of hosts that BTV infects as appears to be the case for influenza virus (Finkelstein et al., 2007).
Acknowledgments
This work was partly supported by the Biotechnology and Biological Sciences Research Council, UK and partly by National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
References
- Ahnert P, Patel SS. Asymmetric interactions of hexameric bacteriophage T7 DNA helicase with the 5′- and 3′-tails of the forked DNA substrate. J Biol Chem. 1997;272:32267–32273. doi: 10.1074/jbc.272.51.32267. [DOI] [PubMed] [Google Scholar]
- Appleby TC, Luecke H, Shim JH, Wu JZ, Cheney IW, Zhong W, Vogeley L, Hong Z, Yao N. Crystal structure of complete rhinovirus RNA polymerase suggests front loading of protein primer. J Virol. 2005;79:277–288. doi: 10.1128/JVI.79.1.277-288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerjee AK, Shatkin AJ. Transcription in vitro by reovirus associated ribonucleic acid-dependent polymerase. J Virol. 1970;6:1–11. doi: 10.1128/jvi.6.1.1-11.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa PR, Sousa MO, Barbosa EC, Bomfim Ade S, Ginefra P, Nadal J. Analysis of the prevalence of ventricular late potentials in the late phase of myocardial infarction based on the site of infarction. Arq Bras Cardiol. 2002;78:352–363. doi: 10.1590/s0066-782x2002000400002. [DOI] [PubMed] [Google Scholar]
- Bartlett NM, Gillies SC, Bullivant S, Bellamy AR, Lawton JA, Zeng CQ, Mukherjee SK, Cohen J, Estes MK, Prasad BV, Smith RE, Furuichi Y, Gillies S, Bullivant S, Bellamy AR. Electron microscopy study of reovirus reaction cores. J Virol. 1974;14:315–326. doi: 10.1128/jvi.14.2.315-326.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss CD, Smith GL. Vaccinia virion protein I8R has both DNA and RNA helicase activities: implications for vaccinia virus transcription. J Virol. 1996;70:794–800. doi: 10.1128/jvi.70.2.794-800.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton AR, Rodriguez J, Reddy YK, Roy P. The membrane trafficking protein calpactin forms a complex with bluetongue virus protein NS3 and mediates virus release. Proc Natl Acad Sci U S A. 2002;99:13154–13159. doi: 10.1073/pnas.192432299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya B, Noad RJ, Roy P. Interaction between Bluetongue virus outer capsid protein VP2 and vimentin is necessary for virus egress. Virol J. 2007 Jan 15;4:7. doi: 10.1186/1743-422X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird LE, Brannigan JA, Subramanya HS, Wigley DB. Characterisation of Bacillus stearothermophilus PcrA helicase: evidence against an active rolling mechanism. Nucleic Acids Res. 1998;26:2686–2693. doi: 10.1093/nar/26.11.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Roy P. Recovery of infectious bluetongue virus from RNA. J Virol. 2007;81:2179–2186. doi: 10.1128/JVI.01819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Celma CC, Roy P. A Reverse genetics system for Bluetongue virus. Journal of Virology. 2008 doi: 10.1128/JVI.00808-08. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Wehrfritz J, Noad R, Roy P. Purified recombinant bluetongue virus VP1 exhibits RNA replicase activity. J Virol. 2004;78:3994–4002. doi: 10.1128/JVI.78.8.3994-4002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn JA. Relationships among the positive strand and double-strand RNA viruses as viewed through their RNA-dependent RNA polymerases. Nucleic Acids Res. 1991;19:217–226. doi: 10.1093/nar/19.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn JA. A structural and primary sequence comparison of the viral RNA-dependent RNA polymerases. Nucleic Acids Res. 2003;31:1821–1829. doi: 10.1093/nar/gkg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujnicki JM, Rychlewski L. Reassignment of specificities of two cap methyltransferase domains in the reovirus lambda 2 protein. Genome Biol. 2001;2:RESEARCH0038. doi: 10.1186/gb-2001-2-9-research0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher SJ, MGJ, Makeyev EV, Bamford DH, Stuart DI. A mechanism for initiating RNA-dependent RNA polymerization. Nature. 2001;410:235–240. doi: 10.1038/35065653. [DOI] [PubMed] [Google Scholar]
- Chen D, Zeng CQ, Wentz MJ, Gorziglia M, Estes MK, Ramig RF. Template-dependent, in vitro replication of rotavirus RNA. J Virol. 1994;68:7030–7039. doi: 10.1128/jvi.68.11.7030-7039.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Groarke JM, Young DC, Kuhn RJ, Smith JL, Pevear DC, Rossmann MG. The structure of the RNA-dependent RNA polymerase from bovine viral diarrhea virus establishes the role of GTP in de novo initiation. Proc Natl Acad Sci U S A. 2004;101:4425–4430. doi: 10.1073/pnas.0400660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman PM, Lawrence MC. The structural biology of type I viral membrane fusion. Nat Rev Mol Cell Biol. 2003;4:309–319. doi: 10.1038/nrm1076. [DOI] [PubMed] [Google Scholar]
- de la Cruz J, Kressler D, Linder P. Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem Sci. 1999;24:192–198. doi: 10.1016/s0968-0004(99)01376-6. [DOI] [PubMed] [Google Scholar]
- DEFRA VSCT. Report On The Distribution Of Bluetongue Infection In Great Britain On 15 March 2008. DEFRA; 2008. http://www.defra.gov.uk/animalh/diseases/notifiable/bluetongue/pdf/epi-report080508.pdf. [Google Scholar]
- Dillingham MS, Kowalczykowski SC. A step backward in advancing DNA replication: rescue of stalled replication forks by RecG. Mol Cell. 2001;8:734–736. doi: 10.1016/s1097-2765(01)00358-6. [DOI] [PubMed] [Google Scholar]
- Diprose JM, Burroughs JN, Sutton GC, Goldsmith A, Gouet P, Malby R, Overton I, Zientara S, Mertens PP, Stuart DI, Grimes JM. Translocation portals for the substrates and products of a viral transcription complex: the bluetongue virus core. Embo J. 2001;20:7229–7239. doi: 10.1093/emboj/20.24.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton BT, Hyatt AD. Association of bluetongue virus with the cytoskeleton. Subcell Biochem. 1989;15:233–273. doi: 10.1007/978-1-4899-1675-4_8. [DOI] [PubMed] [Google Scholar]
- Eaton BT, Hyatt AD, Brookes SM. The replication of bluetongue virus. Curr Top Microbiol Immunol. 1990;162:89–118. doi: 10.1007/978-3-642-75247-6_4. [DOI] [PubMed] [Google Scholar]
- EFSA. Epidemiological analysis of the 2006 bluetongue virus serotype 8 epidemic in north-western Europe. European Food Safety Authority; 2007. http://www.efsa.europa.eu/EFSA/1178620925100/efsa_locale-1178620753812_Bluetongue.htm. [Google Scholar]
- Egloff MP, Benarroch D, Selisko B, Romette JL, Canard B. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. Embo J. 2002;21:2757–2768. doi: 10.1093/emboj/21.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus BJ. The history of bluetongue. Prog Clin Biol Res. 1985;178:7–12. doi: 10.1016/b978-012369368-6.50006-x. [DOI] [PubMed] [Google Scholar]
- Espejo RT, Lopez S, Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981;37:156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes MK, Graham DY, Mason BB. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981;39:879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrega C, Hausmann S, Shen V, Shuman S, Lima CD. Structure and mechanism of mRNA cap (guanine-N7) methyltransferase. Mol Cell. 2004;13:77–89. doi: 10.1016/s1097-2765(03)00522-7. [DOI] [PubMed] [Google Scholar]
- Ferrer-Orta C, Arias A, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. Structure of foot-and-mouth disease virus RNA-dependent RNA polymerase and its complex with a template-primer RNA. J Biol Chem. 2004;279:47212–47221. doi: 10.1074/jbc.M405465200. [DOI] [PubMed] [Google Scholar]
- Finkelstein DB, Mukatira S, Mehta PK, Obenauer JC, Su X, Webster RG, Naeve CW. Persistent host markers in pandemic and H5N1 influenza viruses. J Virol. 2007;81:10292–10299. doi: 10.1128/JVI.00921-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forzan M, Wirblich C, Roy P. A capsid protein of nonenveloped Bluetongue virus exhibits membrane fusion activity. Proc Natl Acad Sci U S A. 2004;101:2100–2105. doi: 10.1073/pnas.0306448101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forzan M, M M, Roy P. Bluetongue virus entry into the cells. Journal of Virology. 2007;81:4819–4827. doi: 10.1128/JVI.02284-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts ET, Yu X, Egelman EH, Botchan MR. Biochemical and electron microscopic image analysis of the hexameric E1 helicase. J Biol Chem. 1999;274:4447–4458. doi: 10.1074/jbc.274.7.4447. [DOI] [PubMed] [Google Scholar]
- French TJ, Roy P. Synthesis of bluetongue virus (BTV) corelike particles by a recombinant baculovirus expressing the two major structural core proteins of BTV. J Virol. 1990;64:1530–1536. doi: 10.1128/jvi.64.4.1530-1536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French TJ, Inumaru S, Roy P. Expression of two related nonstructural proteins of bluetongue virus (BTV) type 10 in insect cells by a recombinant baculovirus: production of polyclonal ascitic fluid and characterization of the gene product in BTV-infected BHK cells. J Virol. 1989;63:3270–3278. doi: 10.1128/jvi.63.8.3270-3278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst TR, Fernandez MP, Moss B. Transfer of the inducible lac repressor/operator system from Escherichia coli to a vaccinia virus expression vector. Proc Natl Acad Sci U S A. 1989;86:2549–2553. doi: 10.1073/pnas.86.8.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukusho A, Yu Y, Yamaguchi S, Roy P. Completion of the sequence of bluetongue virus serotype 10 by the characterization of a structural protein, VP6, and a non-structural protein, NS2. J Gen Virol. 1989;70:1677–1689. doi: 10.1099/0022-1317-70-7-1677. [DOI] [PubMed] [Google Scholar]
- Furuichi Y, Muthukrishnan S, Shatkin A. 5′-Terminal m-7G(5′)ppp(5′)G-m-p in vivo: identification in reovirus genome RNA. Proc Natl Acad Sci U S A. 1975;72:742–745. doi: 10.1073/pnas.72.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S, Bullivant S, Bellamy AR. Viral RNA polymerases: electron microscopy of reovirus reaction cores. Science. 1971;174:694–696. doi: 10.1126/science.174.4010.694. [DOI] [PubMed] [Google Scholar]
- Gogol EP, Seifried SE, von Hippel PH. Structure and assembly of the Escherichia coli transcription termination factor rho and its interaction with RNA. I. Cryoelectron microscopic studies. J Mol Biol. 1991;221:1127–1138. doi: 10.1016/0022-2836(91)90923-t. [DOI] [PubMed] [Google Scholar]
- Gouet P, Diprose JM, Grimes JM, Malby R, Burroughs JN, Zientara S, Stuart DI, Mertens PP. The highly ordered double-stranded RNA genome of bluetongue virus revealed by crystallography. Cell. 1999;97:481–490. doi: 10.1016/s0092-8674(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Jakana J, Ghosh M, Basak AK, Roy P, Chiu W, Stuart DI, Prasad BVV. An atomic model of the outer layer of the bluetongue virus core derived from X-ray crystallography and electron cryomicroscopy. Structure. 1997;5:885–893. doi: 10.1016/s0969-2126(97)00243-8. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Burroughs JN, Gouet P, Diprose JM, Malby R, Zientara S, Mertens PPC, Stuart DI. The atomic structure of the bluetongue virus core. Nature. 1998;395:470–478. doi: 10.1038/26694. [DOI] [PubMed] [Google Scholar]
- Gross CH, Shuman S. Mutational analysis of vaccinia virus nucleoside triphosphate phosphohydrolase II, a DExH box RNA helicase. J Virol. 1995;69:4727–4736. doi: 10.1128/jvi.69.8.4727-4736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson K, Doherty AJ, Shuman S, Wigley DB. X-ray crystallography reveals a large conformational change during guanyl transfer by mRNA capping enzymes. Cell. 1997;89:545–553. doi: 10.1016/s0092-8674(00)80236-6. [DOI] [PubMed] [Google Scholar]
- Hansen JL, Long AM, Schultz SC. Structure of the RNA-dependent RNA polymerase of poliovirus. Structure. 1997;5:1109–1122. doi: 10.1016/s0969-2126(97)00261-x. [DOI] [PubMed] [Google Scholar]
- Hassan SH, Wirblich C, Forzan M, Roy P. Expression and functional characterization of bluetongue virus VP5 protein: role in cellular permeabilization. J Virol. 2001;75:8356–8367. doi: 10.1128/JVI.75.18.8356-8367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewat EA, Booth TF, Roy P. Structure of bluetongue virus particles by cryoelectron microscopy. J Struct Biol. 1992a;109:61–69. doi: 10.1016/1047-8477(92)90068-l. [DOI] [PubMed] [Google Scholar]
- Hewat EA, Booth TF, Roy P. Structure of correctly self-assembled bluetongue virus-like particles. J Struct Biol. 1994;112:183–191. doi: 10.1006/jsbi.1994.1019. [DOI] [PubMed] [Google Scholar]
- Hewat EA, Booth TF, Loudon PT, Roy P. Three-dimensional reconstruction of baculovirus expressed bluetongue virus core-like particles by cryo-electron microscopy. Virology. 1992b;189:10–20. doi: 10.1016/0042-6822(92)90676-g. [DOI] [PubMed] [Google Scholar]
- Hodel AE, Gershon PD, Quiocho FA. Structural basis for sequence-nonspecific recognition of 5′-capped mRNA by a cap-modifying enzyme. Mol Cell. 1998;1:443–447. doi: 10.1016/s1097-2765(00)80044-1. [DOI] [PubMed] [Google Scholar]
- Huismans H, Verwoerd DW. Control of transcription during the expression of the bluetongue virus genome. Virology. 1973;52:81–88. doi: 10.1016/0042-6822(73)90400-5. [DOI] [PubMed] [Google Scholar]
- Huismans H, Els HJ. Characterization of the tubules associated with the replication of three different orbiviruses. Virology. 1979;92:397–406. doi: 10.1016/0042-6822(79)90144-2. [DOI] [PubMed] [Google Scholar]
- Huismans H, Van Dijk AA, Els HJ. Uncoating of parental bluetongue virus to core and subcore particles in infected L cells. Virology. 1987;157:180–188. doi: 10.1016/0042-6822(87)90327-8. [DOI] [PubMed] [Google Scholar]
- Hyatt AD, Zhao Y, Roy P. Release of bluetongue virus-like particles from insect cells is mediated by BTV nonstructural protein NS3/NS3A. Virology. 1993;193:592–603. doi: 10.1006/viro.1993.1167. [DOI] [PubMed] [Google Scholar]
- Kadare G, Haenni AL. Virus-encoded RNA helicases. J Virol. 1997;71:2583–2590. doi: 10.1128/jvi.71.4.2583-2590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar AK, Roy P. Defining the structure-function relationships of bluetongue virus helicase protein VP6. J Virol. 2003;77:11347–11356. doi: 10.1128/JVI.77.21.11347-11356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar AK, Ghosh M, Roy P. Mapping the assembly of Bluetongue virus scaffolding protein VP3. Virology. 2004;324:387–399. doi: 10.1016/j.virol.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Kar AK, Bhattacharya B, Roy P. Bluetongue virus RNA binding protein NS2 is a modulator of viral replication and assembly. BMC Mol Biol. 2007;8:4. doi: 10.1186/1471-2199-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Antar AA, Boehme KW, Danthi P, Eby EA, Guglielmi KM, Holm GH, Johnson EM, Maginnis MS, Naik S, Skelton WB, Wetzel JD, Wilson GJ, Chappell JD, Dermody TS. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe. 2007;1:147–157. doi: 10.1016/j.chom.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoto S, Sasaki J, Taniguchi K. Reverse genetics system for introduction of site-specific mutations into the double-stranded RNA genome of infectious rotavirus. Proc Natl Acad Sci U S A. 2006;103:4646–4651. doi: 10.1073/pnas.0509385103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakkonen P, Hyvonen M, Peranen J, Kaariainen L. Expression of Semliki Forest virus nsP1-specific methyltransferase in insect cells and in Escherichia coli. J Virol. 1994;68:7418–7425. doi: 10.1128/jvi.68.11.7418-7425.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blois H, French T, Mertens PP, Burroughs JN, Roy P. The expressed VP4 protein of bluetongue virus binds GTP and is the candidate guanylyl transferase of the virus. Virology. 1992;189:757–761. doi: 10.1016/0042-6822(92)90600-t. [DOI] [PubMed] [Google Scholar]
- Lecatsas G. Electron microscopic study of the formation of bluetongue virus. Onderstepoort J Vet Res. 1968;35:139–149. [PubMed] [Google Scholar]
- Li C, Xia Y, Gao X, Gershon PD. Mechanism of RNA 2′-O-methylation: evidence that the catalytic lysine acts to steer rather than deprotonate the target nucleophile. Biochemistry. 2004;43:5680–5687. doi: 10.1021/bi0359980. [DOI] [PubMed] [Google Scholar]
- Lin C, Kim JL. Structure-based mutagenesis study of hepatitis C virus NS3 helicase. J Virol. 1999;73:8798–8807. doi: 10.1128/jvi.73.10.8798-8807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman TM, Thom K, Vale RD. Staying on track: common features of DNA helicases and microtubule motors. Cell. 1998;93:9–12. doi: 10.1016/s0092-8674(00)81139-3. [DOI] [PubMed] [Google Scholar]
- Loudon PT, Roy P. Assembly of five bluetongue virus proteins expressed by recombinant baculoviruses: inclusion of the largest protein VP1 in the core and virus-like proteins. Virology. 1991;180:798–802. doi: 10.1016/0042-6822(91)90094-r. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos K, Wirblich C, Brierley I, Roy P. Sequence specificity in the interaction of Bluetongue virus non-structural protein 2 (NS2) with viral RNA. J Biol Chem. 2003;278:31722–31730. doi: 10.1074/jbc.M301072200. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos K, Noad R, Tosi S, Nethisinghe S, Brierley I, Roy P. Specific binding of Bluetongue virus NS2 to different viral plus-strand RNAs. Virology. 2006;353:17–26. doi: 10.1016/j.virol.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, Bamford DH. Replicase activity of purified recombinant protein P2 of double-stranded RNA bacteriophage phi6. EMBO J. 2000a;19:124–133. doi: 10.1093/emboj/19.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, Bamford DH. The polymerase subunit of a dsRNA virus plays a central role in the regulation of viral RNA metabolism. EMBO J. 2000b;19:6275–6284. doi: 10.1093/emboj/19.22.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, Bamford DH. Primer-independent RNA sequencing with bacteriophage phi6 RNA polymerase and chain terminators. RNA. 2001;5:774–781. doi: 10.1017/s1355838201002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SA, Moss B. mRNA guanylyltransferase and mRNA (guanine-7-)-methyltransferase from vaccinia virions. Donor and acceptor substrate specificites. J Biol Chem. 1976;251:7313–7321. [PubMed] [Google Scholar]
- Martin SA, Paoletti E, Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975;250:9322–9329. [PubMed] [Google Scholar]
- Martinez Costas J, Sutton G, Ramadevi N, Roy P. Guanylyltransferase and RNA 5′-triphosphatase activities of the purified expressed VP4 protein of bluetongue virus. J Mol Biol. 1998;280:859–866. doi: 10.1006/jmbi.1998.1926. [DOI] [PubMed] [Google Scholar]
- McDougal VV, Guarino LA. DNA and ATP binding activities of the baculovirus DNA helicase P143. J Virol. 2001;75:7206–7209. doi: 10.1128/JVI.75.15.7206-7209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens PP, Brown F, Sangar DV. Assignment of the genome segments of bluetongue virus type 1 to the proteins which they encode. Virology. 1984;135:207–217. doi: 10.1016/0042-6822(84)90131-4. [DOI] [PubMed] [Google Scholar]
- Mertens PP, Burroughs JN, Anderson J. Purification and properties of virus particles, infectious subviral particles, and cores of bluetongue virus serotypes 1 and 4. Virology. 1987;157:375–386. doi: 10.1016/0042-6822(87)90280-7. [DOI] [PubMed] [Google Scholar]
- Mertens PPC, Maan S, Samuel A, Attoui H. Orbivirus, Reoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy, VIIIth Report of the ICTV. London: Elsevier/Academic Press; 2005. pp. 466–483. [Google Scholar]
- Mi S, Durbin R, Huang HV, Rice CM, Stollar V. Association of the Sindbis virus RNA methyltransferase activity with the nonstructural protein nsP1. Virology. 1989;170:385–391. doi: 10.1016/0042-6822(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Modrof J, Lymperopoulos K, Roy P. Phosphorylation of Bluetongue Virus Nonstructural Protein 2 Is Essential for Formation of Viral Inclusion Bodies. J Virol. 2005;79:10023–10031. doi: 10.1128/JVI.79.15.10023-10031.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason E, Rothnagel R, Muknerge SK, Kar AK, Forzan M, Prasad BVV, Roy P. Interactions between the Inner and Outer Capsids of Bluetongue Virus. J Virol. 2004;78:8059–8067. doi: 10.1128/JVI.78.15.8059-8067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KS, Cherney MM, Lopez Vazquez A, Machin A, Martin Alonso JM, Parra F, james MNG. Crystal Structures of active and inactive conformations of a caliciviral RNA-dependent RNA polymerase. Journal of Biological Chemistry. 2002;277:1381–1387. doi: 10.1074/jbc.M109261200. [DOI] [PubMed] [Google Scholar]
- Nibert ML, Schiff LA, Fields BN. Mammalian reoviruses contain a myristoylated structural protein. J Virol. 1991;65:1960–1967. doi: 10.1128/jvi.65.4.1960-1967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noad R, Roy P. Bluetongue virus assembly and morphogenesis. In: Roy P, editor. Current Topics in Microbiology and Immunology. Berlin: Heidelberg; New York: Springer-Verlag; 2006. pp. 87–116. [DOI] [PubMed] [Google Scholar]
- O’Reilly EK, Kao CC. Analysis of RNA-dependent RNA polymerase structure and function as guided by known polymerase structures and computer predictions of secondary structure. Virology. 1998;252:287–303. doi: 10.1006/viro.1998.9463. [DOI] [PubMed] [Google Scholar]
- Owens R, Roy P. Role of an Arbovirus Nonstructural Protein in Cellular Pathogenesis and Virus Release. J Virol. 2004;78:6649–6656. doi: 10.1128/JVI.78.12.6649-6656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini C, De Francesco R, Gallinari P. Enzymatic properties of hepatitis C virus NS3-associated helicase. J Gen Virol. 2000;81:1335–1345. doi: 10.1099/0022-1317-81-5-1335. [DOI] [PubMed] [Google Scholar]
- Parker R, Song H. The enzymes and control of eukaryotic mRNA turnover. Nat Struct Mol Biol. 2004;11:121–127. doi: 10.1038/nsmb724. [DOI] [PubMed] [Google Scholar]
- Patton JT. Rotavirus VP1 alone specifically binds to the 3′ end of viral mRNA, but the interaction is not sufficient to initiate minus-strand synthesis. J Virol. 1996;70:7940–7947. doi: 10.1128/jvi.70.11.7940-7947.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JT, Chen D. RNA-binding and capping activities of proteins in rotavirus open cores. J Virol. 1999;73:1382–1391. doi: 10.1128/jvi.73.2.1382-1391.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JT, Jones MT, Kalbach AN, He YW, Xiaobo J. Rotavirus RNA polymerase requires the core shell protein to synthesize the double-stranded RNA genome. J Virol. 1997;71:9618–9626. doi: 10.1128/jvi.71.12.9618-9626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesavento JB, Crawford SE, Estes MK, Prasad BV. Rotavirus proteins: structure and assembly. Curr Top Microbiol Immunol. 2006;309:189–219. doi: 10.1007/3-540-30773-7_7. [DOI] [PubMed] [Google Scholar]
- Piccininni S, Varaklioti A, Nardelli M, Dave B, Raney KD, McCarthy JE. Modulation of the hepatitis C virus RNA-dependent RNA polymerase activity by the non-structural (NS) 3 helicase and the NS4B membrane protein. J Biol Chem. 2002;277:45670–45679. doi: 10.1074/jbc.M204124200. [DOI] [PubMed] [Google Scholar]
- Prasad BVV, Yamaguchi S, Roy P. Three-dimensional structure of single-shelled bluetongue virus. J Virol. 1992;66:2135–2142. doi: 10.1128/jvi.66.4.2135-2142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purse BV, Mellor PS, Rogers DJ, Samuel AR, CMPP, Baylis M. Climate Change And The Recent Emergence Of Bluetongue In Europe. Nature Reviews Microbiology. 2005;3:171–181. doi: 10.1038/nrmicro1090. [DOI] [PubMed] [Google Scholar]
- Ramadevi N, Rodriguez J, Roy P. A leucine zipper-like domain is essential for dimerization and encapsidation of bluetongue virus nucleocapsid protein VP4. J Virol. 1998a;72:2983–2990. doi: 10.1128/jvi.72.4.2983-2990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadevi N, Burroughs JN, Mertens PPC, Jones IM, Roy P. Capping and methylation of mRNA by purified recombinant VP4 protein of Bluetongue virus. Proc Natl Acad Sci USA. 1998b;95:13537–13542. doi: 10.1073/pnas.95.23.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R, Singh R, Shimba S. Methylated cap structures in eukaryotic RNAs: structure, synthesis and functions. Pharmacol Ther. 1992;54:249–267. doi: 10.1016/0163-7258(92)90002-h. [DOI] [PubMed] [Google Scholar]
- Reinisch KM, Nibert ML, Harrison SC. Structure of the reovirus core at 3.6 A resolution. Nature. 2000;404:960–967. doi: 10.1038/35010041. [DOI] [PubMed] [Google Scholar]
- Roner MR, Joklik WK. Reovirus reverse genetics: Incorporation of the CAT gene into the reovirus genome. Proc Natl Acad Sci U S A. 2001;98:8036–8041. doi: 10.1073/pnas.131203198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman F, Shatkin AJ, Perry RP. Sequences containing methylated nucleotides at the 5′ termini of messenger RNAs: possible implications for processing. Cell. 1974;3:197–199. doi: 10.1016/0092-8674(74)90131-7. [DOI] [PubMed] [Google Scholar]
- Roy P. Bluetongue virus proteins. J Gen Virol. 1992;73:3051–3064. doi: 10.1099/0022-1317-73-12-3051. [DOI] [PubMed] [Google Scholar]
- Roy P. Orbiviruses and their replication. In: Fields BN, editor. Field’s Virology. 3. Philadelphia: Lippincott-Raven; 1995. pp. 1709–1734. [Google Scholar]
- Roy P. Bluetongue virus proteins and their role in virus entry, assembly and release. In: Roy P, editor. Virus Structure and Assembly. USA: Elsevier Academic Press; 2005. pp. 69–114. [Google Scholar]
- Roy P. Orbiviruses and their replication. In: Knipe DM, Howley PM, editors. Fields’ Virology. 5. Philadelphia, New York, USA: Lippincott Williams & Wilkins; 2007. pp. 1975–1997. [Google Scholar]
- Roy P, Marshall JJ, French TJ. Structure of the bluetongue virus genome and its encoded proteins. Current Topics in Microbiology and Immunology. 1990a;162:43–87. doi: 10.1007/978-3-642-75247-6_3. [DOI] [PubMed] [Google Scholar]
- Roy P, Fukusho A, Ritter GD, Lyon D. Evidence for genetic relationship between RNA and DNA viruses from the sequence homology of a putative polymerase gene of bluetongue virus with that of vaccinia virus: conservation of RNA polymerase genes from diverse species. Nucleic Acids Res. 1988;16:11759–11767. doi: 10.1093/nar/16.24.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P, Adachi A, Urakawa T, Booth TF, Thomas CP. Identification of bluetongue virus VP6 protein as a nucleic acid-binding protein and the localization of VP6 in virus-infected vertebrate cells. Journal of Virology. 1990b;64:1–8. doi: 10.1128/jvi.64.1.1-8.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Potterton L, Yuan F, van Vlijmen H, Karplus M. Evaluation of comparative protein modeling by MODELLER. Proteins. 1995;23:318–326. doi: 10.1002/prot.340230306. [DOI] [PubMed] [Google Scholar]
- Schmid SR, Linder P. D-E-A-D protein family of putative RNA helicases. Mol Microbiol. 1992;6:283–291. doi: 10.1111/j.1365-2958.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Sedman J, Stenlund A. The papillomavirus E1 protein forms a DNA-dependent hexameric complex with ATPase and DNA helicase activities. J Virol. 1998;72:6893–6897. doi: 10.1128/jvi.72.8.6893-6897.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A, Both G. Reovirus mRNA: transcription and translation. Cell. 1976;7:305–313. doi: 10.1016/0092-8674(76)90159-8. [DOI] [PubMed] [Google Scholar]
- Shi J, Blundell TL, Mizuguchi K. FUGUE: sequence-structure homology recognition using environment-specific substitution tables and structure-dependent gap penalties. J Mol Biol. 2001;310:243–257. doi: 10.1006/jmbi.2001.4762. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Luo H, Qin W, Kaneko S, Yamashita T, Kobayashi K, Murakami S. Hepatitis C virus (HCV) NS5A binds RNA-dependent RNA polymerase (RdRP) NS5B and modulates RNA-dependent RNA polymerase activity. J Biol Chem. 2002;277:11149–11155. doi: 10.1074/jbc.M111392200. [DOI] [PubMed] [Google Scholar]
- Silverstein SC, Schonberg M, Levin DH, Acs G. The reovirus replicative cycle: conservation of parental RNA and protein. Proc Natl Acad Sci U S A. 1970;67:275–281. doi: 10.1073/pnas.67.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Sidwell RW, Clark SM, Barnett BB, Spendlove RS. Inhibition of bluetongue and Colorado tick fever orbiviruses by selected antiviral substances. Antimicrob Agents Chemother. 1981;20:533–538. doi: 10.1128/aac.20.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes MC, Joklik WK. Reovirus protein lambda 3 is a poly(C)-dependent poly(G)polymerase. Virology. 1993;193:356–366. doi: 10.1006/viro.1993.1132. [DOI] [PubMed] [Google Scholar]
- Stauber N, Martinez-Costas J, Sutton G, Monastyrskaya K, Roy P. Bluetongue virus VP6 protein binds ATP and exhibits an RNA-dependent ATPase function and a helicase activity that catalyze the unwinding of double-stranded RNA substrates. J Virol. 1997;71:7220–7226. doi: 10.1128/jvi.71.10.7220-7226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart DI, Grimes JM. Structural studies on orbivirus proteins and particles. Curr Top Microbiol Immunol. 2006;309:221–244. doi: 10.1007/3-540-30773-7_8. [DOI] [PubMed] [Google Scholar]
- Sutton G, Grimes JM, Stuart DI, Roy P. Bluetongue virus VP4 is an RNA-capping assembly line. Nature Struct Mol Biol. 2007;14:449–451. doi: 10.1038/nsmb1225. [DOI] [PubMed] [Google Scholar]
- Tai CL, Pan WC, Liaw SH, Yang UC, Hwang LH, Chen DS. Structure-based mutational analysis of the hepatitis C virus NS3 helicase. J Virol. 2001;75:8289–8297. doi: 10.1128/JVI.75.17.8289-8297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Farsetta DL, Nibert ML, Harrison SC. RNA Synthesis in a Cage-Structural Studies of Reovirus Polymerase lambda3. Cell. 2002;111:733–745. doi: 10.1016/s0092-8674(02)01110-8. [DOI] [PubMed] [Google Scholar]
- Thomas CP, Booth TF, Roy P. Synthesis of bluetongue virus-encoded phosphoprotein and formation of inclusion bodies by recombinant baculovirus in insect cells: it binds the single-stranded RNA species. J Gen Virol. 1990;71:2073–2083. doi: 10.1099/0022-1317-71-9-2073. [DOI] [PubMed] [Google Scholar]
- Tortorici MA, Broering TJ, Nibert ML, Patton JT. Template recognition and formation of initiation complexes by the replicase of a segmented double-stranded RNA virus. J Biol Chem. 2003;3:3. doi: 10.1074/jbc.M305358200. [DOI] [PubMed] [Google Scholar]
- Urakawa T, Roy P. Bluetongue virus tubules made in insect cells by recombinant baculoviruses: expression of the NS1 gene of bluetongue virus serotype 10. J Virol. 1988;62:3919–3927. doi: 10.1128/jvi.62.11.3919-3927.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa T, Ferguson M, Minor PD, Cooper J, Sullivan M, Almond JW, Bishop DH. Synthesis of immunogenic, but non-infectious, poliovirus particles in insect cells by a baculovirus expression vector. Journal of General Virology. 1989;70:1453–1463. doi: 10.1099/0022-1317-70-6-1453. [DOI] [PubMed] [Google Scholar]
- Urushibara T, Furuichi Y, Nishimura C, Miura K. A modified structure at the 5′-terminus of mRNA of vaccinia virus. FEBS Lett. 1975;49:385–389. doi: 10.1016/0014-5793(75)80791-5. [DOI] [PubMed] [Google Scholar]
- Van Dijk AA, Huismans H. The in vitro activation and further characterization of the bluetongue virus-associated transcriptase. Virology. 1980;104:347–356. doi: 10.1016/0042-6822(80)90339-6. [DOI] [PubMed] [Google Scholar]
- Van Dijk AA, Huismans H. The effect of temperature on the in vitro transcriptase reaction of bluetongue virus, epizootic haemorrhagic disease virus and African horsesickness virus. Onderstepoort J Vet Res. 1982;49:227–232. [PubMed] [Google Scholar]
- Van Dijk AA, Huismans H. In vitro transcription and translation of bluetongue virus mRNA. J Gen Virol. 1988;69:573–581. doi: 10.1099/0022-1317-69-3-573. [DOI] [PubMed] [Google Scholar]
- Venkatesan S, Gershowitz A, Moss B. Modification of the 5′ end of mRNA. Association of RNA triphosphatase with the RNA guanylyltransferase-RNA (guanine-7-)methyltransferase complex from vaccinia virus. J Biol Chem. 1980;255:903–908. [PubMed] [Google Scholar]
- Verwoerd DW, Huismans H. Studies on the in vitro and the in vivo transcription of the bluetongue virus genome. Onderstepoort J Vet Res. 1972;39:185–191. [PubMed] [Google Scholar]
- Verwoerd DW, Louw H, Oellermann RA. Characterization of bluetongue virus ribonucleic acid. J Virol. 1970;5:1–7. doi: 10.1128/jvi.5.1.1-7.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd DW, Els HJ, De Villiers EM, Huismans H. Structure of the bluetongue virus capsid. J Virol. 1972;10:783–794. doi: 10.1128/jvi.10.4.783-794.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrfritz JM, Boyce M, Mirza S, Roy P. Reconstitution of bluetongue virus polymerase activity from isolated domains based on a three-dimensional structural model. Biopolymers. 2007;86:83–94. doi: 10.1002/bip.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell. 1975;4:379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- White PW, Pelletier A, Brault K, Titolo S, Welchner E, Thauvette L, Fazekas M, Cordingley MG, Archambault J. Characterization of recombinant HPV6 and 11 E1 helicases: effect of ATP on the interaction of E1 with E2 and mapping of a minimal helicase domain. J Biol Chem. 2001;276:22426–22438. doi: 10.1074/jbc.M101932200. [DOI] [PubMed] [Google Scholar]
- Wirblich C, Bhattacharya B, Roy P. Nonstructural protein 3 of bluetongue virus assists virus release by recruiting ESCRT-I protein Tsg101. Journal of Virology. 2006;80:460–473. doi: 10.1128/JVI.80.1.460-473.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Chen SY, Iwata H, Compans RW, Roy P. Multiple glycoproteins synthesized by the smallest RNA segment (S10) of bluetongue virus. J Virol. 1992;66:7104–7112. doi: 10.1128/jvi.66.12.7104-7112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Walker SB, Chipman PR, Nibert ML, Baker TS. Reovirus polymerase lambda3 localized by cryo-electron microscopy of virions at a resolution of 7.6 A. Nat Struct Biol. 2003;10:1011–1018. doi: 10.1038/nsb1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tang J, Walker SB, O’Hara D, Nibert ML, Duncan R, Baker TS. Structure of avian orthoreovirus virion by electron cryomicroscopy and image reconstruction. Virology. 2005;343:25–35. doi: 10.1016/j.virol.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]