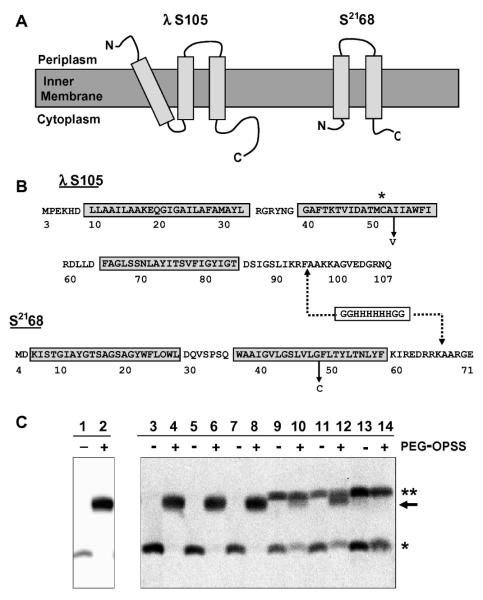
Figure 1.
(A) Topologies of holin proteins. S105 and S2168 are inner membrane proteins with three or two TMDs, respectively. (B) Sequences of holin proteins. S105 begins with the Met residue from codon 3 of the S gene [15]. S2168 begins with Met at position 4 of S21 [9]. The TMDs are indicated by boxes, and the relevant mutations A52V and G48C, for S105 and S2168, respectively, are labeled. The asterisk highlights the position of the Cys residue in S105. The dashed lines indicate the position of the oligohistidine tag inserted in each sequence: between positions 94 and 95 in S105 [2] and between positions 66 and 67 in S2168 [9]. For simplicity, the his-tagged lambda holin is called S105 in the text, although it actually corresponds to the protein S105τ94 [2] where τ94 refers to the position at which the oligo-his tag was inserted (C) Western blot for samples from PEGylation of S105A52V purification, detected with antibodies against peptides corresponding to the C-terminus of S105 [7]. Lanes 1 and 2, TCA-precipitated whole cells expressing S105A52V; lanes 3 and 4, cell suspension; lanes 5 and 6, French pressate; lanes 7 and 8, clearing spin supernatant; lanes 9 and 10, resuspended membranes; lanes 11 and 12, membrane extraction spin supernatant; lanes 13 and 14, purified S105A52V (* indicates the S monomer band, ** indicates the S dimer band, and → indicates the PEGylation product).