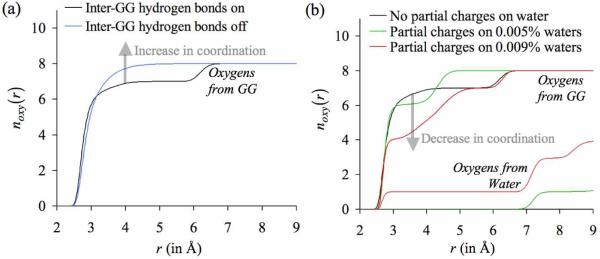
Figure 7.
Effect of altering the densities of hydrogen bond donors in ΓC on the number distributions of oxygens noxy(r) around K+. The final configuration of the simulation with four glycine dipeptide (GG) molecules in the simulation box (n GG = 4 case in figure 6) was used as the starting configuration for two separate sets of simulations: one in which the densities of hydrogen bond donors were decreased, and another in which the densities of hydrogen bond donors were increased. (a) Since the oxygen atoms of GG molecules showed a tendency to hydrogen bond with the amino groups in adjacent GG molecules, the densities of hydrogen bond donors were effectively reduced by turning-off all inter-GG hydrogen bond interactions. This increased the number of oxygens directly coordinating the K+ ion, as indicated by a transparent arrow. (b) The densities of hydrogen bond donors were increased by turning on the natural partial charges of a certain percentage of water molecules in the simulation box. This decreased the total number of oxygen atoms directly coordinating the K+ ion, as also indicated by a transparent arrow. Note that in the case where the partial charges on 0.009% of the waters are turned on, this reduction in coordination number occurs despite one oxygen atom from water substituting for the oxygen atoms from the GG molecule.