Abstract
Polymorphisms in the RGS2 (regulator of G-protein signaling 2) gene were found to be associated with anxious behavior in mice and anxiety in humans. We examined whether rs4606, a single nucleotide polymorphism (SNP) of RGS2, and social support moderated risk for PTSD in an epidemiologic sample. The study examines 607 adults from the 2004 Florida Hurricanes study who returned buccal DNA samples via mail. Rs4606 was associated with increased symptoms of post-hurricane PTSD symptoms under conditions of high hurricane exposure and low social support (p<0.05). Further, this polymorphism was associated with lifetime PTSD symptoms under conditions of lifetime exposure to a potentially traumatic event, and low social support (p<0.001). These gene by environment interactions remained significant after adjustment for sex, ancestry, and age. RGS2 rs4606 modifies risk of post-disaster and lifetime PTSD symptoms under conditions of high stressor exposure. This is the first demonstration of gene-environment interaction for this locus.
Keywords: Posttraumatic Stress Disorder, RGS2, genetic by environment interaction, candidate gene, trauma
Potentially traumatic events (PTE), such as disasters, physical and sexual abuse or assault, motor vehicle accident, or unexpected/sudden death of a loved one, occur frequently and embody a distinctive set of stressors that have effects on many levels such as basic needs, functional impairment, physical health, and mental health (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995; Kilpatrick et al., 2003; Norris et al., 2002; Resnick, Kilpatrick, Dansky, Saunders, & Best, 1993). A growing literature has investigated the psychiatric correlates of PTEs (Galea, Nandi, & Vlahov, 2005; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995; Kilpatrick et al., 2003; Norris et al., 2002; Resnick, Kilpatrick, Dansky, Saunders, & Best, 1993), generally concluding that PTE exposure increases the risk for a variety of psychiatric phenotypes including posttraumatic stress disorder [PTSD] and that risk is affected by a number of factors, such as severity of exposure (Acierno et al., in press), interpersonal victimization (Resnick, Kilpatrick, Dansky, Saunders, & Best, 1993), and lack of social support (Brewin, Andrews, & Valentine, 2000) (for review see Brewin, Andrews, & Valentine, 2000). Further, most individuals who experience traumatic stressors never develop PTSD (Bonanno, 2004), and it is therefore also important to elucidate mechanisms of risk and resilience. Although considerable effort has been given to identify variables (e.g., individual difference variables, aspects of the environment) that are related to risk, a high percentage of variance remains unexplained (Bonanno, 2004). Following, there has been a growing interest in examining genetic determinates of PTSD (Lee et al., 2005; Zhang et al., 2006); however, modern studies of genetic risk factors for PTSD remain few in number.
Most extant genetic research is guided by a “main effects” model that examines direct effects of either genotype or environment upon manifestation of mental disorders; in fact, out of the 11 case-control candidate gene studies conducted to date on PTSD, 9 have used this model, with mixed success (Koenen, 2008). As noted by Moffitt and colleagues “ … it seems reasonable to suggest that whenever there is variation among human’s psychological reactions to a major environmental pathogen for mental disorder, [gene-environment interactions] must be expected to some degree” (p.473; Moffitt, Caspi, & Rutter, 2005). The gene by environment (GxE) interaction model posits that the effects of environmental stressors on mental disorder phenotypes are moderated by genotype. In contrast to the “main effect” model, the interaction model proposed by Moffitt and colleagues (Moffitt, Caspi, & Rutter, 2005) provides a new paradigm for the study of phenotypic expression that is highly suited for PTSD research, given that a criteria of the disorder is exposure to an environmental stressor.
Two GxE studies of PTSD have been published. Binder and colleagues found that childhood sexual abuse interacted with FKPB5 to predict PTSD in adults (Binder et al., 2008). Kilpatrick and colleagues’ (Kilpatrick et al., 2007) analyses of the 5-HTTLPR polymorphism replicated, and extended to PTSD, Caspi and colleagues’ (Caspi et al., 2003) model of a GxE effect, specifically the interaction between environmental variables (severity of disaster exposure, level of social support) and genotype afforded more power in the prediction of major depression and PTSD than did the main effect analyses. The present study examines an additional gene, regulator of G-protein signaling 2 (RGS2), utilizing the same sample.
G-protein coupled receptors comprise one of the largest groups of signaling proteins (Hollmann, Strumper, Serroeder, & Durieux, 2005). G-protein-coupled receptors are extracellular ligand proteins that alert the cell to certain changes in the intercellular environment through changes in their conformation (Hollinger & Helper, 2002). First, the binding induces an alteration in the conformation of the receptor. Second, coupling and activation of one (or more) G-proteins occurs. Third, these G-proteins then regulate events in the intercellular environment. G-proteins get their name because their primary action is with interactions of the guanine nucleotide (Hollmann, Strumper, Serroeder, & Durieux, 2005). G-protein activation signals a cascade in vascular smooth muscle cell receptors that causes in increase in Ca2+ which then leads to vasoconstriction (Hollinger & Helper, 2002). Regulators of G-protein signaling bind to Gα subunits and increase their GTPase activity which then attenuates downstream signaling (Hollinger & Helper, 2002). RGS2, a potent regulator that reduces G-protein activity, selectively inhibits Gqα (Heximer, 2004), thereby increasing GTPase activity and decreasing vasoconstriction (and lowering blood pressure).
The RGS2 gene has been associated with anxiety in animal models and in human correlational studies. RGS2 knockout mice, both hetero- and homozygous, have increased hypertension (Heximer et al., 2003), increased excitability in CA1 neurons in the hippocampus (Oliviera-dos-Santos et al., 2000), and anxiety (Yalcin et al., 2004). A targeted genome screen found modest evidence for linkage between markers including RGS2 and anxiety disorder proneness (Smoller et al., 2001). Recently, Legraf, and colleagues (Leygraf et al., 2006) found that polymorphisms in RGS2 were associated with panic disorder in humans, with the strongest association being observed for a haplotype containing SNPs rs4606 (C as risk allele) and rs3767488. Smoller and colleagues (2008) also found significant associations between rs4606 and anxiety-related temperament, personality, and brain function (Smoller et al., 2008), but this paper found G to be the risk allele. Cui and colleagues found a significant difference in the distribution of rs4606 genotypes in suicide victims versus controls (Cui et al., 2007). Variation in rs4606 is of particular interest because it is associated with variation in RGS2 mRNA expression (Semplicini et al., 2006).
The present study examined the association between rs4606, a polymorphism in RGS2, and PTSD in an epidemiologic sample of adults exposed to the 2004 Florida hurricanes. Following previous research (Leygraf et al., 2006), we hypothesized that the C allele, in comparison to the G allele, of RGS2 would increase risk for PTSD symptoms under conditions of high environmental stress (high disaster/PTE exposure and low social support).
Method
Materials & Methods
Data Collection and Sample
This study examines data from 607 participants in the 2004 Florida Hurricanes Study who completed structured telephone interviews and provided saliva samples that yielded genotype data for the rs4606 polymorphism. Methodological details for the Florida Hurricanes Study are provided elsewhere (Acierno et al., 2007; Acierno, Ruggiero, Kilpatrick, Resnick, & Galea, 2006; Galea et al., 2006; Kilpatrick et al., 2007). Demographic characteristics of the 607 sample participants were as follows: 64.9% women; 35.1% men; 22.6% ≤ 59 years old, 77.4% ≥ 60 years old; 90% European-American (EA), 3.9% African-American (AA), 3.9% Hispanic, 1.7% other, 0.5% missing self-report race/ethnicity data.
Participants provided verbal consent and they were sent letters documenting the elements of verbal consent and providing them with contact information for the principal investigator. Participants received $20 for completion of the interview and saliva sample.
Assessment Procedure
A probability sample of adults from telephone households in 38 counties in Florida within 6 9 months of the 2004 hurricane season, between April 5 and June 12, 2005 completed telephone interviews. Sample selection and telephone interviewing via random-digit-dial procedures was performed by Schulman, Ronca, Bucuvalas, Inc., an experienced research firm (Galea et al., 2002). Assessments were conducted via highly structured interview using computer-assisted telephone interview (CATI) methodology that allows for considerable quality control.
PTSD was assessed with the National Women’s Study (NWS) PTSD module, an often used measure in population-based research. This module has concurrent validity and reliability (e.g., temporal stability, internal consistency, diagnostic reliability; Ruggiero, Rheingold, Resnick, Kilpatrick, & Galea, 2006). The NWS PTSD module was validated in the DSM-IV PTSD field trial against the Structured Clinical Interview for DSM (SCID), yielding an interrater kappa coefficient of 0.85 for the diagnosis of PTSD and comparisons between scores on the National Women’s Study PTSD module and the SCID yielded a kappa coefficient of 0.71 for current and 0.77 for lifetime PTSD (Kilpatrick et al., 1998). Research also has found considerable correspondence between telephone versus in-person administration of this module (Acierno, Resnick, Kilpatrick, & Stark-Riemer, 2003). We assessed both lifetime PTSD symptoms and PTSD symptoms since the hurricanes, both of which had excellent reliability (.86, .87, respectively).
Stressor exposure (hurricane exposure for post-hurricane PTSD, potentially traumatic event [PTE] exposure for lifetime PTSD) and social support were previously found to be associated with PTSD symptom level in this sample (Acierno et al., 2007) and were therefore included as covariates in the current regression models of post-hurricane symptom count.
Hurricane exposure was assessed with indicators identified in previous research (Freedy, Saladin, Kilpatrick, Resnick, & Saunders, 1994) on the basis of relation to posthurricane psychological functioning: (1) being personally present during hurricane-force winds or major flooding; (2) one week or more of lack of adequate access to food, water, electricity, telephone, or clothing; (3) losses or significant damage in two or more of five predefined categories of hurricane-related loss (i.e., furniture; meaningful possessions; automobile; pets; crops, trees, garden); (4) one week or longer home displacement; and (5) un-reimbursed losses of $1,000 or more. High hurricane exposure was defined as having experienced two or more of these indicators (45.8% of sample).
PTE exposure was measured using behaviorally specific language that asked if participants had been exposed to five types of events and during this exposure feared that they would be killed or seriously injured: (1) natural disaster (other than 2004 hurricanes); (2) serious work accident; (3) attacked with a gun; (4) attacked without a weapon; and (5) military combat or being in a war zone.
Social support during the six months prior to the hurricanes was measured via a modified version of the Medical Outcomes Study module (Sherbourne & Stewart, 1991) that assesses emotional, instrumental, and appraisal social support with five items (sample range=0–20; mean=15.9, SD=4.8). A score of 15 or less (37.0% of the sample) was considered “low social support” based on the cutoff score derived from prior work (Galea et al., 2002). This scale had excellent reliability (Chronbach’s alpha=0.86).
Collection of DNA samples
A mouthwash protocol was used to obtain saliva samples that were returned via mail to the Yale University laboratory for DNA extraction and analyses. Samples were provided by 651 participants (42.2% response rate). Valid genetic ancestry data were available for 623 cases (95.7%), and valid genotype data were available for 607 cases (93.2%). The likelihood of submitting a saliva sample did not differ in relation to key variables (i.e., hurricane exposure, sex, social support, PTSD symptoms). Details on response rate and associations of participation are described elsewhere (Galea et al., 2006).
Genotyping
PUREGENE kits (Gentra Systems, Minneapolis) were used to extract DNA from saliva. Rs 4606 was genotyped with a fluorogenic 5’ nuclease assay method (“TaqMan”) via the ABI PRISM 7900 Sequence Detection System (ABI, Foster City, CA, USA). Genotypes were assayed twice and discordant genotypes were discarded.
Further, 36 markers were genotyped to yield ancestry information (Stein, Schork, & Gelernter, 2004; Yang, Zhao, Kranzler, & Gelernter, 2005a, 2005b). One additional highly informative SNP marker, SLC24A5 (Lamason et al., 2005), was added to the panel.
Ancestry Proportion Scores
Ancestry proportion scores were created to control for spurious associations that can occur from variation in allele frequency and prevalence of trait by population. Bayesian cluster analysis was used to estimate participants’ ancestries with the marker panel described above on the procedures and STRUCTURE software developed by Falush, Stephens, and Pritchard, (2003) and Pritchard and Rosenberg (1999). For the STRUCTURE analysis, the “admixture” and “allele frequencies correlated” models were specified and we utilized 100,000 burn-in and 100,000 Markov chain Monte Carlo iterations.
Statistical Analyses
The prevalence of post-hurricane PTSD diagnosis in this sample was 3.6%. Due to the relatively low prevalence of PTSD and to maximize power for interaction analyses, we used symptom counts as our outcome variable. We used correlational analyses to test whether the rs4606 polymorphism in RGS2 was associated with PTSD symptoms. Chi-square analyses determined if genotype was related to any of the stressor variables. Linear regression analyses determined whether any observed association persisted after adjusting for sex, age, ancestral proportion scores, social support and stressor exposure. To determine whether the association between RGS2 and PTSD was modified by level of stress exposure, we tested all higher order interactions between RGS2 genotype and stress exposure variables (social support and hurricane exposure for post-hurricane symptoms, social support and PTE exposure for lifetime symptom count).
Results
Average number of post-hurricane PTSD symptoms reported among those who provided valid genotype data for rs4606 in RGS2 (N=607) was 1.62 (SD=2.76), and the average number of lifetime PTSD symptoms reported was 3.16 (SD=3.61). Thirty-seven percent of individuals (N=224) endorsed low social support, and was associated with post-hurricane and lifetime PTSD symptoms (rs=.24, .28, respectively, ps<.001). Similarly, stressor exposure was associated with PTSD symptom counts. High hurricane exposure was reported by 44.6% of participants with valid RGS2 data (N=271), and was related to posthurricane PTSD symptoms (r=.14, p=.001). PTE exposure was reported by 45% of participants (N=273), and was related to lifetime PTSD symptoms (r=.40, p<.001).
RGS2 SNP rs4606 genotype frequencies were in Harvey-Weinberg equilibrium. Genotype frequencies were similar to those reported by Leygraf et al (2006): GG (n=47, 7.7%), CG (n=221, 36.4%), and CC (n=339, 55.8%). Genotype frequency did not differ by self-reported racial/ethnic groups (χ2 =.82, df=1, ns).
As expected, there was no association between RGS2 genotype and either exposure variable (low social support: χ2 =.03, df=1, ns; high hurricane exposure: χ2 =1.44, df=1, ns; PTE exposure: χ2 =.12, df=1, ns).
Ancestral proportion scores were weakly correlated with post-hurricane symptom count, r =.09, p<.05, and therefore ancestral proportion was controlled for in regression analyses. There was no significant correlation between ancestral proportion score and lifetime PTSD symptom count, r =.06, ns, thus, even without correction based on ancestry coefficients, population stratification is not a potential cause of false positive findings for lifetime count.
The final regression models for post-hurricane PTSD symptoms and lifetime PTSD symptoms are shown in Table 1. Neither of the two way interactions (genotype by hurricane exposure; genotype by social support) was a significant predictor of post-hurricane or lifetime PTSD symptoms. The three way interaction (genotype by hurricane exposure by social support), however, was significantly predictive in both models (post-hurricane PTSD symptoms: β=.58, t=2.18, p=.029; lifetime PTSD symptoms: β=1.12, t=3.71, p<.001) (see Figure 1). The same pattern of results was found when conducting these analyses with the sample restricted to Caucasian participants, and when controlling for the effects of the 5HTTLPR genotype.
Table 1.
Final Linear Regression Analysis of the Effects of Hurricane Exposure, Social Support, and the RGS2 genotype on Posthurricane and Lifetime PTSD Symptoms
| Post-hurricane PTSD Symptoms |
Lifetime PTSD Symptoms |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | β | b | t | p | β | b | t | p |
| Sex | .03 | .18 | .77 | .44 | .11 | .83 | 3.07 | .002 |
| Age less than 60 years | .09 | .59 | 2.14 | .03 | .10 | .82 | 2.55 | .01 |
| Ancestral proportion score | .04 | .59 | 1.01 | .31 | .00 | -.05 | -.07 | .94 |
| Stressor (hurricane or PTE) | .06 | .34 | 1.24 | .21 | .28 | 2.03 | 6.44 | <.001 |
| Low social support | .16 | .93 | 3.21 | <.001 | .11 | .86 | 2.47 | .01 |
| RGS2 genotype | .01 | .02 | .13 | .89 | .00 | .02 | .08 | .93 |
| RGS2 by stressor by social support | .26 | .58 | 2.18 | .03 | .20 | 1.12 | 3.71 | <.001 |
Figure 1. Lifetime PTSD Symptoms by Risk Group.
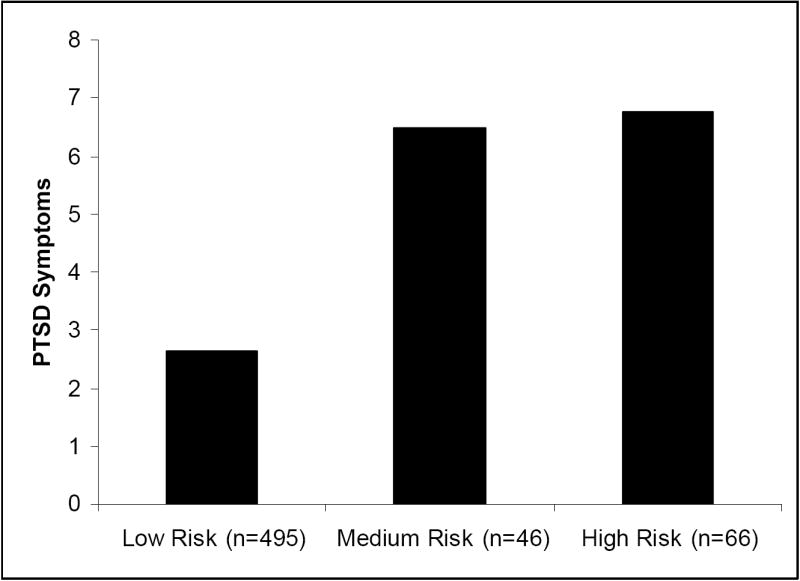
Note: Low risk group is those with ‘G/G’ genotype, high social support, and low PTE. Medium risk group is those with ‘C/G’ genotype, low social support, and high PTE. High risk group is those with ‘C/C’ genotype, low social support, high PTE. This is the same pattern found for post-hurricane symptoms.
Discussion
Our results demonstrate that rs4606, a polymorphism in the RGS2 gene, moderates the risk of PTSD symptom severity under high environmental stress conditions of low social support and high hurricane exposure (or PTE). To our knowledge, this is the first study to date that examines RGS2 through a GxE model in a population based human sample in relation to PTSD. Consistent with Leygraf and colleagues (Leygraf et al., 2006) but (in terms of the identity of the associated allele) not with Smoller and colleagues (2008), the ‘C’ allele was associated with increased risk of anxiety, but in the current study, this was found only under stress conditions. Similar to past research (Leygraf et al., 2006; Smoller et al., 2008) the present study found that this polymorphism is only accounting for a small amount of the variance in symptoms. This highlights that PTSD is a complex phenotype, and that most likely there are many genetic and environmental factors that confer risk for the disorder (Koenen, Nugent, & Amstadter, 2008). Nonetheless, identifying that a variant in this gene is associated with increased risk of symptoms in those exposed to stressful environmental factors (disaster/PTE, low social support) advances the literature. From a pharmacological standpoint, RGS2 modulators may afford innovative pharmacological avenues for treatment of anxiety disorders (Smoller et al., 2008).
The current study has limitations. This cross-sectional data was collected 6-9 months following hurricanes and therefore retrospective recall bias may exist for not only the lifetime exposure variables, but also post-disaster variables. DNA samples were returned for less than one half of the sample. However, there were no significant differences between returners and non-returners of saliva samples.
In sum, our results are consistent with both animal (Oliviera-dos-Santos et al., 2000) and human studies (Leygraf et al., 2006) implicating RGS2 in the etiology of anxiety disorders. However, the biological mechanism by which the variation in RGS2 interacts with stressors to confer risk for PTSD remains to be elucidated. The present study extends the literature by examining a new phenotype, PTSD, and by the analysis of environmental interactions. Our research underscores the need to assess environmental stressor variables. We cannot provide a fully satisfactory explanation for observation of association of opposite alleles with anxiety-related traits in different studies. Some possible explanations are (a) the differences in phenotype account for the differences in association; and (b) the marker studied is in linkage disequilibrium with some different functional variant, and the direction of LD differs by population. Future research that undertakes fine mapping of the 3’ region of RGS2 are warranted to further our knowledge of its role in anxiety disorders. Future research should also examine endophenotypes that are likely to be more closely related to genetic variants than phenotypes (Gottesman & Gould, 2003).
Acknowledgments
This research was supported by NIMH grant MH05220 (principal investigator: Ron Acierno, Ph.D.). Ananda Amstadter is supported by NIMH 18869. Dr. Koenen is supported by NIMH grants K08-MH070627 and MH078928.
Footnotes
The authors declare no conflicting financial or other competing interests.
References
- Acierno R, Resnick H, Kilpatrick DG, Stark-Riemer W. Assessing elder victimization: demonstration of a methodology. Social Psychiatry and Psychiatric Epidemiology. 2003;38:644–653. doi: 10.1007/s00127-003-0686-4. [DOI] [PubMed] [Google Scholar]
- Acierno R, Ruggiero KJ, Galea S, Resnick HS, Koenen K, Roitzsch J, de Arellano M, Boyle J, Kilpatrick DG. Psychological sequelae resulting from the 2004 Florida hurricanes: implications for postdisaster intervention. American Journal of Public Health. 2007;97(Suppl 1):S103–108. doi: 10.2105/AJPH.2006.087007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acierno R, Ruggiero KJ, Kilpatrick DG, Resnick HS, Galea S. Risk and protective factors for psychopathology among older versus younger adults after the 2004 Florida hurricanes. American Journal of Geriatric Psychiatry. 2006;14:1051–1059. doi: 10.1097/01.JGP.0000221327.97904.b0. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Journal of the American Medical Association. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA. Loss, trauma, and human resilience. Have we underestimated the human capacity to thrive after extremely aversive events? American Psychologist. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology. 2000;68:317–336. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig I, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5- HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cui H, Nishiguchi N, Ivleva E, Yanagi M, Fukutake M, Nushida H, et al. Association of RGS2 gene polymorphisms with suicide and increased RGS2 immunoreactivity in the postmortem brain of suicide victims. Neuropsychopharmacology. 2007;33:1537–1544. doi: 10.1038/sj.npp.1301557. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedy JR, Saladin ME, Kilpatrick DG, Resnick HS, Saunders BE. Understanding acute psychological distress following natural disaster. Journal of Traumatic Stress. 1994;7:257–273. doi: 10.1007/BF02102947. [DOI] [PubMed] [Google Scholar]
- Galea S, Acierno R, Ruggiero K, Resnick H, Tracy M, Kilpatrick DG. Social context and the psychobiology of posttraumatic stress. Annals of the New York Academy of Sciences. 2006;1071:231–241. doi: 10.1196/annals.1364.018. [DOI] [PubMed] [Google Scholar]
- Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, et al. Psychological sequelae of the September 11 terrorist attacks in New York city. New England Journal of Medicine. 2002;346:982–987. doi: 10.1056/NEJMsa013404. [DOI] [PubMed] [Google Scholar]
- Galea S, Nandi A, Vlahov D. The epidemiology of post-traumatic stress disorder after disasters. Epidemiology Review. 2005;27:78–91. doi: 10.1093/epirev/mxi003. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Heximer SP. RGS2-mediated regulation of Gqalpha. Methods in Enzymology. 2004;390:65–82. doi: 10.1016/S0076-6879(04)90005-5. [DOI] [PubMed] [Google Scholar]
- Heximer SP, Knutsen RH, Sun X, Kaltenbronn KM, Rhee MH, Peng N, et al. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. Journal of Clinical Investigation. 2003;111:445–452. doi: 10.1172/JCI15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger S, Helper JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacology Review. 2002;54:527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Hollmann MW, Strumper D, Serroeder S, Durieux ME. Receptors, G proteins, and their interactions. Anestesiology. 2005;103:1066–1078. doi: 10.1097/00000542-200511000-00022. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Freedy JR, Pelcovitz D, Resick PA, Roth S, et al. The posttraumatic stress disorder field trial: evaluation of the PTSD construct—criteria A through E. In: Widiger T, Pincus HA, First MB, Ross R, Davis W, editors. DSM-IV Sourcebook. Washington, DC: American Psychiatric Press; 1998. pp. 803–844. [Google Scholar]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. Journal of Consulting and Clinical Psychology. 2003;71:692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- Koenen KC. Genetics of posttraumatic stress disorder: review and recommendations for future studies. Journal of Traumatic Stress. 2007;20:737–750. doi: 10.1002/jts.20205. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Nugent NR, Amstadter AB. Posttraumatic stress disorder: A review and agenda for gene-environment interaction research in trauma. European Archives of Psychiatry and Clinical Neuroscience. 2008;258:82–96. doi: 10.1007/s00406-007-0787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamason RL, Mohideen MA, Mest JR, Wong AC, Norton HL, Aros MC, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee MS, Kang RH, Kim H, Kim SD, Kee BS, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depression and Anxiety. 2005;21:135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- Leygraf A, Hohoff C, Freitag C, Willis-Owen SAG, Krakowitzky P, Fritze J, et al. Rgs 2 gene polymorphisms as modulators of anxiety in humans? Journal of Neural Transmission. 2006;113:1921–1925. doi: 10.1007/s00702-006-0484-8. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 Disaster Victims Speak: Part 1 An Empirical Review of the Empirical Literature 1981-2001. Psychiatry. 2002;65(3):207–239. doi: 10.1521/psyc.65.3.207.20173. [DOI] [PubMed] [Google Scholar]
- Oliviera-dos-Santos AJ, Matsumoto G, Snow BE, Bai D, Houston FP, Whishaw IQ, et al. Regulation of T cell activation, anxiety, and male aggression by RGS2. Proceedings of the National Academy of Sciences. 2000;97:12272–12277. doi: 10.1073/pnas.220414397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. American Journal of Human Genetics. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. Journal of Consulting and Clinical Psychology. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Ruggiero KJ, Rheingold AA, Resnick HS, Kilpatrick DG, Galea S. Comparison of two widely used PTSD-screening instruments: implications for public mental health planning. Journal of Traumatic Stress. 2006;19:699–707. doi: 10.1002/jts.20141. [DOI] [PubMed] [Google Scholar]
- Semplicini A, Lenzini L, Sartori M, Papparella I, Calò LA, Pagnin E, et al. Reduced expression of regulator of G-protein signaling 2 (RGS2) in hypertensive patients increases calcium mobilization and ERK1/2 phosphorylation induced by angiotensin II. Journal of Hypertension. 2006;24:1115–1124. doi: 10.1097/01.hjh.0000226202.80689.8f. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS Social Support Survey. Social Science and Medicine. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Acierno JS, Jr, Rosenbaum JF, Biederman J, Pollack MH, Meminger S, et al. Targeted genome screen of panic disorder and anxiety disorder proneness using homology to murine QTL regions. American Journal of Medical Genetics. 2001;105:195–206. doi: 10.1002/ajmg.1209. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Paulus MP, Fagerness JA, Purcell S, HYamaki LH, Hirshfeld-Becker D, et al. RGS2 influences anxiety-related temperament, personality and brain function. Archives of General Psychiatry. 2008;65:298–308. doi: 10.1001/archgenpsychiatry.2007.48. [DOI] [PubMed] [Google Scholar]
- Stein MB, Schork NJ, Gelernter J. A polymorphism of the beta1-adrenergic receptor is associated with low extraversion. Biological Psychiatry. 2004;56:217–224. doi: 10.1016/j.biopsych.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Yalcin B, Willis-Owen SAG, Fullerton J, Meesaq A, Deacon RM, Rawlins JNP, et al. Genetic dissection of a behavioral quantitative train locus shows that Rgs2 modulates anxiety in mice. Nature Genetics. 2004;36:1197–11202. doi: 10.1038/ng1450. [DOI] [PubMed] [Google Scholar]
- Yang BZ, Zhao H, Kranzler HR, Gelernter J. Characterization of a Likelihood Based Method and Effects of Markers Informativeness in Evaluation of Admixture and Population Group Assignment. BMC Genetics. 2005a;6:50. doi: 10.1186/1471-2156-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BZ, Zhao H, Kranzler HR, Gelernter J. Practical population group assignment with selected informative markers: Characteristics and properties of Bayesian clustering via STRUCTURE. Genetic Epidemiology. 2005b;28:302–312. doi: 10.1002/gepi.20070. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, Charney DS, et al. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. American Journal of Medical Genetics: Part B Neuropsychiatric Genetics. 2006;141:387–393. doi: 10.1002/ajmg.b.30332. [DOI] [PMC free article] [PubMed] [Google Scholar]


