Abstract
Purpose
Radiofrequency ablation (RFA) is a common treatment modality for surgically unresectable tumors. However, there is a high rate of both local and systemic recurrence.
Experimental Design
In this pre-clinical study, we sought to enhance the antitumor effect of RFA by combining it with huKS-IL2 immunocytokine (tumor-specific monoclonal antibody fused to interleukin 2) in mice bearing CT26-KS colon adenocarcinoma. Mice were treated with RFA, huKS-IL2 via intratumoral injection, or combination therapy.
Results
Treatment of mice bearing subcutaneous tumors with RFA and huKS-IL2 resulted in significantly greater tumor growth suppression and enhanced survival, compared to mice treated with RFA or huKS-IL2 alone. When subtherapeutic regimens of RFA or huKS-IL2 were used, tumors progressed in all treated mice. In contrast, the combination of RFA and immunocytokine resulted in complete tumor resolution in 50% of mice. Treatment of a tumor with RFA and intratumoral huKS-IL2 also demonstrated antitumor effects against a distant untreated tumor. Tumor-free mice following treatment with RFA and huKS-IL2 demonstrated immunological memory based on their ability to reject subsequent challenges of CT26-KS and the more aggressive parental CT26 tumors. Flow cytometry analysis of tumor-reactive T-cells from mice with complete tumor resolution demonstrated that treatment with RFA and huKS-IL2 resulted in a greater proportion of cytokine-producing CD4 T-cells and CD8 T-cells compared to mice treated with RFA or huKS-IL2 alone.
Conclusions
These results show that the addition of huKS-IL2 to RFA significantly enhances the antitumor response in this murine model, resulting in complete tumor resolution and induction of immunological memory.
Keywords: radiofrequency ablation, immunocytokine, colon cancer, IL2
Introduction
Although surgical resection remains the most effective treatment for both primary and metastatic liver tumors, only 10–20% of the roughly 37,000 patients who present each year with colorectal liver metastases or primary liver tumors are candidates for liver resection, due to multifocal disease, tumor location near key vascular or biliary structures, or low hepatic reserve 1–7. Radiofrequency ablation (RFA) is the most common ablative cancer treatment in clinical practice for tumors that are surgically unresectable. RFA destroys tumor cells by transmitting electrical current from a radiofrequency generator to a probe tip placed directly in the tumor via image-guidance or direct visualization. This high frequency (400 kHz) alternating current causes rapid ionic vibrations within tissue, creating heat through friction and tumor cell destruction by thermal coagulative necrosis 8,9. This treatment modality is most commonly used for primary or metastatic liver tumors, but its application has been broadened to primary tumors located in the breast, bone, brain, thyroid, parathyroid, kidney and lung 6,10–12. The benefits of using an ablative treatment such as RFA include low morbidity, the potential use of minimally invasive approaches such as laparoscopy and percutaneous methods instead of open surgery, and repeated treatment in cases of recurrence 2,9,13–15. However, both local and systemic recurrence after RFA treatment have limited the enthusiasm for RFA in this patient population 5,15–18. Therefore, additional treatment strategies are needed.
Immunocytokines (IC) consist of a monoclonal antibody (mAb) covalently linked to one or more cytokine molecules. The huKS-IL2 IC (EMD 273066) is a protein composed of an IgG1 mAb specific for the human Epithelial Cell Adhesion Molecule (EpCAM) antigen, linked to two molecules of interleukin-2 (IL2) 19,20. EpCAM is commonly overexpressed in tumors of epithelial cell origin, including tumors of the colon, prostate, ovary, and pancreas 21–23. ICs, including huKS-IL2, have been shown to target tumors and stimulate an antitumor immune response via IL2 responsive immune cells, as well as immune cells via their Fc receptor 19,24–26. HuKS-IL2 is an experimental immunotherapeutic reagent, now in phase I and II clinical trials27. RFA has also been previously shown in animal and human models to independently induce an immune response against ablated tumor cells without additional immune stimulation 28,29. Since both RFA and IC therapy have been shown to generate antitumor T-cell responses, we hypothesized that these two therapies could act synergistically.
In this study, our objective was to determine if the combination of RFA with huKS-IL2 IC could be synergistic against an EpCAM-expressing tumor. Our aim was to create a clinically relevant model by using RFA in a dose and delivery method that simulates clinical use; namely, inducing local tumor response at treated sites, but followed by local tumor recurrence. We then added localized intratumoral huKS-IL2 IC immunotherapy using a delivery regimen and schedule that we have recently shown induces greater local antitumor efficacy in murine models than systemic administration 25. Specifically, our goals were to determine: a) whether RFA and IC resulted in a synergistic antitumor response based on tumor growth and animal survival, b) whether combination therapy resulted in complete tumor regression, c) whether localized administration of RFA and IC could affect distant tumors, and d) whether combination therapy resulted in an antitumor immune memory response.
Material and Methods
Mice and Anesthetic Agents
Six to eight week old female Balb/c mice were obtained from Harlan-Sprague-Dawley (Madison, WI). All animals were housed in university-approved facilities and were handled according to National Institutes of Health and University of Wisconsin-Madison Research Animal Resource Center guidelines.
Tumor Cells
The CT26 colon adenocarcinoma cell line was grown in DMEM media (Hyclone, Logan, UT) supplemented with penicillin (100U/ml), streptomycin (100 μg/ml), L-glutamine (2 mM) (all from Life Technologies, Inc., Grand Island, NY) and 10% heat-inactivated fetal calf serum (FCS, Sigma Chemicals, St. Louis, MO). Cells were maintained at 37°C in a humidified 5% CO2 atmosphere. The CT26-KS cell line, generated by transfection of the parental CT26 line with the gene encoding human EpCAM, was a gift from R.A. Reisfeld (Scripps Research Institute, La Jolla, CA) and has been described previously 27. Constitutive expression of EpCAM in CT26-KS was maintained by growing the cells in the presence of 1 mg/ml G418 (Hyclone, Logan, UT) in DMEM media as above. Prior to tumor implantation, expression of the EpCAM antigen on the CT26-KS cells was verified by flow cytometry (data not shown). The murine MethA sarcoma cell line was grown in RPMI media (Hyclone, Logan, UT) with additives as described for CT26.
Tumor Models
CT26-KS tumors were induced by injecting mice subcutaneously (s.c.) with 5 × 107 tumor cells in the center of the abdomen. Mice which resolved their initial tumor were monitored for at least 30 days for recurrence before immunological memory was tested in vivo or in vitro. For rechallenge experiments, tumor-free mice were injected s.c. with 5 × 105 CT26 or CT26-KS tumor cells in the contralateral flank distant from the site of their first tumor. Some mice were separately challenged with 2 × 106 Meth A sarcoma cells (a dose that did not result in spontaneous Meth A tumor resolution). For studies of primary and distant tumor effects, mice were implanted with 5 × 105 CT26-KS cells s.c. on the abdomen on day 0, followed by an equivalent dose of CT26-KS tumor cells on day 3 on the flank. In all experiments, tumors were measured with a digital caliper every three days. Tumor volumes were calculated in mm3 using the formula: (length × width × width/2). Data shown are mean tumor volume ± SEM. According to University of Wisconsin animal care guidelines, animals were euthanized with CO2 inhalation when any tumor dimension measured 15 mm or greater.
Radiofrequency Tumor Ablation
RFA was performed using a Radio Therapeutics RF 2000® generator. The RFA probe consisted of a hollow 18 gauge needle containing a wire transducer from an Omega® Type K thermocouple, which facilitated temperature monitoring at the probe tip. After anesthesia was induced, via a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) in 100 μl PBS by intraperitoneal injection, the animal was placed in prone position in the center of an 8 inch square piece of aluminum foil with 5 ml of 0.9% saline to enhance conductivity. An 18 gauge metal injection needle was used as the RFA delivery probe. The probe was inserted into the center of the tumor and an alligator clip from the RF generator was attached. A ground electrode was attached to the foil. Power (in Watts) was controlled manually until target temperatures were reached. Total ablation time and time at target temperature were recorded. Previous studies in both human and animal tissue have demonstrated that RFA works optimally when tissue temperatures are greater than 50°C but less than 110°C. At temperatures greater than 110°C, cavitation and charring severely limit thermal coagulative necrosis 10. Most experiments were done using one of two separate RFA dosing regimens. To simulate the clinical setting of tumor recurrence after RFA, a “partial RFA” strategy using 25 seconds of RFA at 90°C was performed on day 10 or 11 of tumor growth. This resulted in partial ablation (>50% necrosis) of the treated tumors, but 65%–100% recurrence if no additional therapy was administered (data not shown). To compare memory T cell responses in animals which fully resolved their tumors after RFA treatment alone, a “complete RFA” regimen using 40 seconds of RFA at 90°C on day 8 was performed. This resulted in complete eradication of the local tumor, with most animals remaining tumor-free for over 30 days.
HuKS-IL2 IC
HuKS-IL2 IC, originally designated KS1/4-IL2, was a gift from S.D. Gillies (EMD-SeronoLexigen Research Center, Billerica MA) 20,27. It was administered as 5 daily intratumoral (IT) injections of 15 μg (total dose of 75μg), each in 50 μl PBS using a 30 gauge needle directed into the center of the tumor, according to the treatment schedules specified in the text. This dose of IC was previously shown to have antitumor effects when given either by intravenous (IV) or IT injections without toxicity 20,25. Untreated animals served as controls. In experiments designed to investigate a more clinically adaptable IT injection dosing schedule, mice were injected with the same total dose of 75 μg huKS-IL2 given as 2 separate injections of 37.5 μg in 50 μl on days 10 and 14.
Surgical Tumor Resection
In experiments comparing surgical resection with RFA and IC treatment, mice were implanted with 5 × 105 CT26-KS tumor cells on the abdomen and rendered tumor-free with either complete surgical excision on day 11, complete RFA (40 seconds, day 8), or partial RFA (25 seconds, day 11) with huKS-IL2 via IT injection. Mice were anesthetized with ketamine and xylazine per the RFA protocol. S.c tumors were completely excised under sterile conditions and the skin was closed with absorbable suture. Mice were subsequently followed for 30 days for evidence of recurrence prior to tumor rechallenge or sacrifice for flow cytometry.
Flow Cytometry
Flow cytometry was used prior to each in vivo CT26-KS tumor cell implantation to verify expression of EpCAM by the CT26-KS cells (data not shown). Tumor cells were collected and resuspended in PBS with 2% FCS (flow buffer) at a concentration of 5 × 106 cells/ml, and 50 μl of CT26-KS cells were incubated with KS-IL2, 10 μg per 1 × 105 cells at 4°C for 40 min. Cells were washed and stained with a secondary antibody, anti-human IL2-PE (BD Biosciences, San Diego, CA), 2 μg per 1 × 105 cells, for an additional 40 min at 4°C. Staining of cells with secondary Ab only, without IC, was used as a negative control. Cells were washed and analyzed using a FACScan cytofluorometer (Becton Dickinson, San Jose, CA).
Flow cytometry was also used to analyze the population of immune memory T cells present in animals which fully resolved the CT26-KS tumor. Tumor-free mice from RFA, KS-IL2 or combination treatment were followed for 30 days after tumor resolution to monitor for recurrence. These tumor-free mice, as well as naive age-matched controls, were euthanized and spleen cells were obtained. Splenocytes were cultured in vitro for 5 days with gamma-irradiated CT26-KS colon carcinoma or MethA fibrosarcoma cells to provide stimulation with relevant or irrelevant tumor-associated antigen, respectively. To enable cytokine accumulation in endoplasmic reticulum and Golgi, monensin was added to the cell cultures for the last 12 hours. On day 5 of culture, splenocytes were harvested, resuspended at a concentration of 1 × 107 cells/ml, and 50 μl of the cell suspension was stained with anti-CD4-APC or anti-CD8-APC mAbs, followed by fixation and permeabilization of the cells and staining for cytoplasmic GM-CSF and IFN-γ with relevant specific or isotype-matched control mAbs (all antibodies were obtained from BD Pharmingen, CA). Flow cytometry analysis was performed with a BD Calibur flow cytometer (BD Pharmingen, CA) and Cell Quest software (BD Pharmingen). Results are presented as the percentage of CD4+ or CD8+ T cells stained positively with specific GM-CSF or IFN-γ mAb out of the total population of CD4+ or CD8+ T cells.
Statistical Analysis
All statistical calculations were reviewed by Glen E. Leverson, a senior statistician for the UW Dept. of Surgery. A two-tailed Student’s t-test was used to determine the significance of differences between experimental and control values for measures of average tumor growth. Survival curves were generated by the method described by Kaplan and Meier 30. The calculation of significance for the proportion of tumor-free animals was calculated through the Fisher’s Exact Test.
Results
HuKS-IL2 IC and RFA treatment resulted in a synergistic antitumor effect
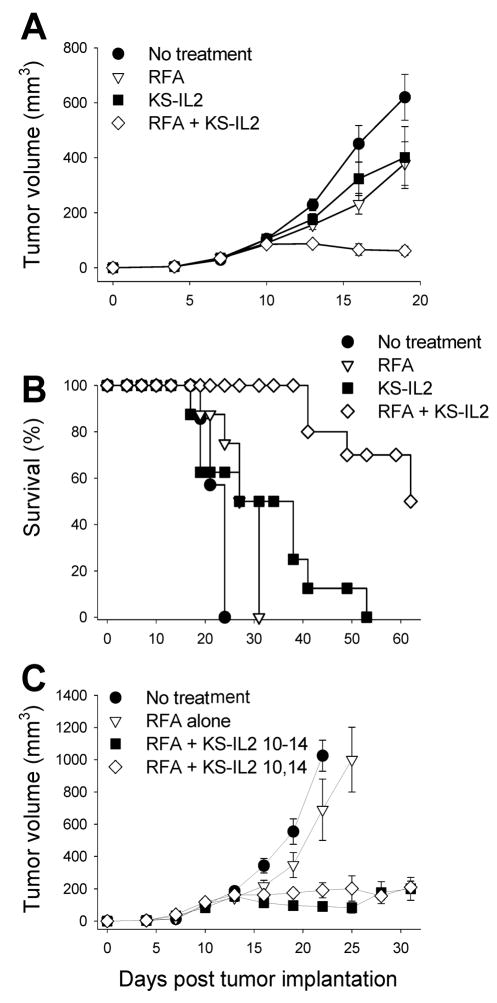
We have previously shown that huKS-IL2 IC has enhanced efficacy when administered by IT injection compared to IV injection 25. Here, we hypothesized that the addition of local IC treatment to partially ablated tumors would result in a greater antitumor effect than either treatment alone. We wanted this murine model to simulate the clinical setting where RFA provides initial local tumor control, but is often followed by local recurrence. By modifying the RFA dose and timing, we developed a “partial RFA” strategy that created a partial ablation of the tumor followed by 65–100% tumor recurrence if no additional therapy was administered. Specifically, treatment of day 11 CT26-KS tumors with 25 seconds of ablation at 90°C resulted in roughly 50–75% necrosis of the tumor. Longer treatment cycles of RFA resulted in variability in tumor growth (data not shown). To test the efficacy of IC combined with RFA, mice bearing CT26-KS tumors were treated with partial RFA, huKS-IL2 IC by IT injection on days 11–15, or RFA and IC used in combination. On day 11, IC was administered at least 60 minutes after RFA treatment in the combination group to allow the tumor bed to cool and prevent destruction of the IC protein. As shown in Figure 1A, treatment with either RFA or huKS-IL2 IC alone resulted in a moderate antitumor effect compared to no treatment (p=0.001 for RFA vs. no treatment and p=0.002 for huKS-IL2 vs. no treatment). Combination therapy with RFA and huKS-IL2 IC resulted in synergistic reduction in tumor growth compared to treatment with RFA alone or IC treatment alone (Fig. 1A, p< 0.001 on day 16; p<0.002 on day 19 for RFA + huKS-IL2 vs. all other groups). These results were confirmed in additional experiments using the same treatment regimens.
Figure 1.
RFA and huKS-IL2 IC synergize in induction of antitumor effects. A, Groups of Balb/c mice (7–8 mice per group) were implanted with 5 × 105 CT26-KS tumor cells s.c. on the abdomen on day 0. Mice were treated with partial RFA (25 seconds, day 11), huKS-IL2 (15 μg, days 11–15), or both RFA and huKS-IL2 IC. Untreated tumor-bearing mice served as controls. Data shown are mean tumor volume ± SEM. B, RFA and huKS-IL2 IC therapy prolongs survival of mice. Balb/c mice bearing CT26-KS tumors were treated as described above and followed until euthanasia due to large tumor size was required. Results in A and B are representative of 3 similar experiments. C, Groups of Balb/c mice (n = 8 mice per group) were implanted with CT26-KS tumors as above and treated with partial RFA alone, RFA and 15 μg huKS-IL2 IC on days 10–14, or RFA and 37.5 μg huKS-IL2 IC on days 10 and 14. Data shown are mean tumor volume ± SEM.
Combination of huKS-IL2 IC and RFA resulted in complete tumor resolution and enhanced survival
Treatment with a combination of RFA and huKS-IL2 IC not only significantly slowed tumor growth, but also resulted in complete tumor resolution in some animals. Using the RFA and IC dosing schedule that resulted in reduced antitumor effects of single treatments shown in Fig. 1A, therapy with RFA plus huKS-IL2 IC led to complete tumor resolution in 8 of 16 mice (in two combined experiments), whereas no mice had resolved tumors in any other group (Table 1, left column). The proportion of tumor-free animals was significant with combination therapy compared to all other treatment groups (p=0.002). Using various RFA and IC dosing schedules, including complete RFA and more therapeutic IC regimens, in four different experiments (data not shown), a proportion of mice treated with either RFA alone or IC alone also had complete tumor resolution. However, the total proportion of tumor-free animals from combination therapy with both RFA and huKS-IL2 remained significantly greater under these conditions as well (Table 1, right column).
Table 1.
Tumor resolution following combined RFA and IC treatment.
Balb/c mice bearing CT26-KS tumors were treated using synergistic treatment conditions: partial RFA alone (25 seconds on day 11), suboptimal IC treatment alone (15μg huKS-IL2 IC on days 11–15), or the combination of these same 2 regimens (RFA + huKS-IL2). Proportions of tumor-free mice to total mice in each treatment group from two combined experiments are shown.
| Treatment Group | Synergistic Conditions | All Experiments |
|---|---|---|
| No treatment | 0/14 | 0/26 |
| RFA alone | 0/14 | 5/26 2 |
| huKS-IL2 alone (days 11–15) | 0/14 | 3/26 3 |
| RFA + huKS-IL2 | 8/161 | 14/284 |
p=0.002 for RFA + huKS-IL2 IC vs. all other groups. When data from all experiments evaluating tumor resolution were combined (right hand column), including some studies with complete RFA, or with more optimal IC schedule administration (see methods), the combined regimen continued to show enhanced tumor resolution. Proportions of tumor-free mice to total mice in each treatment group from 4 combined experiments are shown.
p=0.051 vs. no treatment, p=NS vs. huKS-IL2 alone.
p=NS vs. no treatment, and p=NS vs. RFA alone.
p<0.001 vs. no treatment, p=0.024 vs. RFA alone, p=0.003 vs. huKS-IL2 alone.
Given that the addition of IC to RFA-treated tumors resulted in a significant augmentation of measurable antitumor effects, including complete tumor resolution in some cases, we also determined whether survival differences existed between different treatment groups. With partial RFA and a 5-day course of huKS-IL2 IC, the combination therapy group had significantly enhanced survival compared to untreated animals (p<0.001), RFA alone (p<0.001) and huKS-IL2 IC alone (p=0.002) (Fig. 1B). The survival benefit with combination therapy was reproducible in subsequent experiments. Mice which resolved their tumor were followed for a minimum of 30 additional days to monitor for tumor recurrence. These tumor-free animals underwent tumor rechallenge with a second s.c. injection of tumor cells to test for immunological memory as described below.
Given the potential clinical difficulties required for five daily intratumoral injections of IC to human patients (for example, in treating hepatic metastases), we wished to determine whether this combined regimen might still be effective using fewer IT injections. We therefore tested the response to partial RFA when combined with two concentrated injections of huKS-IL2 IC given on days 10 and 14 (37.5 μg per injection) instead of five injections of 15 μg, thereby maintaining the same total 75 μg dose. When IC was given alone as a single treatment agent, we found that significantly greater antitumor effects occurred with the 5-day course of huKS-IL2 at smaller doses compared to 2 larger doses (data not shown). However, as shown in Figure 1C, when combined with RFA, there was no significant difference between RFA with 2 doses of IC compared to RFA with 5 doses of IC. Notably, both combined treatment regimens were significantly better than RFA alone (p<0.005 on day 25 for RFA alone vs. RFA + huKS-IL2 day 10–14 and day 10 + day 14). Thus, two concentrated doses of huKS-IL2 IC combined with RFA may prove to be an effective treatment regimen, although further studies are required.
RFA and huKS-IL2 resulted in tumor resolution of both treated and distant tumors
Given the ability of RFA, IC, or combined RFA and IC to resolve treated s.c. tumors, we wondered whether this response was strictly a local effect, or whether antitumor effects might also be seen at sites distant to local combined RFA and IC treatment. We tested this using mice bearing two s.c. tumors. Mice were injected s.c. on the abdomen with CT26-KS tumor cells on day 0. A second equivalent tumor inoculation was given on the flank on day 3. Only the abdominal tumor was treated with partial RFA, huKS-IL2 or combined RFA and IC. Growth of both tumors was measured and compared to untreated animals also bearing abdominal and flank tumors. Two consecutive experiments were performed and the pooled results, showing the proportion of animals with complete tumor resolution at abdominal and flank sites, are presented in Table 2. Overall, animals treated with RFA alone had no statistically significant difference in tumor resolution of either the directly treated abdominal tumor or the untreated flank tumor compared to the no treatment group. The RFA and no treatment groups had no resolution of the distant tumors (0/15 mice). Seven of 15 animals treated with huKS-IL2 alone (under these conditions) resolved the treated abdominal tumor, which was significant compared to no treatment (p=0.035) but not compared to RFA alone. However, 8 of 15 animals treated with huKS-IL2 also resolved the untreated flank tumor, which was significant compared to both the RFA and no treatment groups (p= 0.002 vs. RFA or no treatment). The combination of RFA and huKS-IL2 resulted in 14 out of 16 animals with complete resolution of the abdominal tumor, which was significant compared to all other groups. In addition, 13 of 16 animals resolved the untreated flank tumor following combined treatment of the abdominal tumor, which was significant compared to no treatment or RFA groups, although not statistically significant when compared to the huKS-IL2 treatment group (p = 0.10). However, the overall number of tumor-free animals, including both flank and abdominal tumor sites, in the group receiving RFA combined with huKS-IL2 was significant compared to all other groups (p≤0.044). These results indicate that IT administered IC can induce antitumor effects at distant sites, as we have shown previously 25, and also show that IT huKS-IL2 can induce a stronger systemic antitumor effect than RFA. Importantly, combination therapy resulted in a greater proportion of tumor-free mice than either treatment alone.
Table 2.
RFA and huKS-IL2 induced antitumor effects against both treated and distant CT26-KS tumors.
Balb/c mice (7–8 per group) were implanted with 5×105 CT26-KS cells s.c. on the abdomen on day 0. On day 3, an equivalent s.c. CT26-KS tumor cell inoculation was placed on the flank. The abdominal tumor was treated with RFA alone (day 10), 15μg huKS-IL2 IC (days 10–14), or RFA (day 10) and huKS-IL2 IC (days 10–14). The flank tumor remained untreated and was monitored for growth or resolution. Proportions represent animals with complete resolution of either the treated abdominal (left column) or distant tumor (middle column), after pooling two consecutive experiments. Mice which were tumor free at the conclusion of the experiment are listed in the right column.
| Treatment Group | Proportion of mice with no tumor at specific sites | Proportion of tumor-free mice (all sites) | |
|---|---|---|---|
| Treated abdominal tumor | Untreated flank tumor | ||
| No treatment | 1/15 | 0/15 | 0/15 |
| RFA | 5/151 | 0/15 | 0/15 |
| huKS-IL2 | 7/152 | 8/153 | 7/15 6 |
| RFA + huKS- IL2 | 14/164 | 13/165 | 13/16 7 |
p = 0.17 vs. No treatment, and p=NS vs.huKS-IL2;
p = 0.035 vs. no treatment, p = NS vs. RFA;
p = 0.002 vs. no treatment, p = 0.002 vs. RFA;
p <0.001 vs. no treatment, p = 0.023 vs. huKS-IL2, p = 0.003 vs. RFA;
p<0.001 vs. no treatment, p <0.001 vs. RFA, p = 0.14 vs. huKS-IL2. Finally, an analysis of all mice for all sites is included, identifying the proportion of mice that are completely tumor free (right hand column).
p=0.006 vs. no treatment, p = 0.006 vs. RFA ;
p<0.001 vs. no treatment, p <0.001 vs. RFA, p = 0.044 vs. huKS-IL2
The addition of huKS-IL2 to RFA induced tumor-specific immune memory
Modifications in timing or regimen (higher dose and earlier administered RFA, or earlier initiation of 5 days of IC given IT) can enable these individual treatments to eliminate primary CT26-KS tumors in some treated animals. To test if individual or combined treatments induce long-term immunity, mice rendered tumor-free after initial therapy with partial RFA, IT IC, or combined treatment were rechallenged approximately 30 days after resolution of the primary CT26-KS tumor with both CT26-KS cells and parental CT26 cells via s.c. injection in opposite flanks. Table 3A shows the number of animals that rejected CT26-KS and CT26 delayed challenges. In all treatment groups, all animals which had previously rejected a CT26-KS tumor demonstrated a memory response to CT26-KS upon rechallenge, whereas none of the naive control animals rejected this tumor. In addition, Table 3A shows that several animals which received immunotherapy with huKS-IL2 alone or in combination with RFA also were able to reject the parental CT26 tumor challenge. The antitumor response against CT26 tumors in animals treated with both RFA and huKS-IL2 was significantly better than in animals which received RFA alone, but not huKS-IL2 alone (p=0.018 for combined treatment vs. RFA alone; p=0.004 for combined treatment vs. control; p = 0.90 for combined treatment vs. huKS-IL2 alone). This suggests that animals which received IC immune therapy against their primary CT26-KS tumor have an enhanced immune response against tumor antigens found on the CT26 tumor, and are not responding to the foreign, human EpCAM antigen that is present on the CT26-KS tumor.
Table 3.
Immunologic memory after complete resolution of CT26-KS tumors
A: Mice which resolved their primary CT26-KS tumor after treatment with RFA, huKS-IL2 or combination therapy were challenged simultaneously with CT26 (first exposure) and CT26-KS (2nd exposure) on opposite flanks. Proportions represent tumor-free mice in each treatment group.
B: Fifteen mice were implanted with 5×105 CT26-KS tumor cells s.c. on the abdomen on day 0. Mice were then divided into groups and treated with complete tumor ablation on day 8 (n=3 mice, 40 seconds RFA), complete tumor excision (n = 8) on day 11, or partial RFA (25 sec, day 11) with huKS-IL2 on days 11–15 (n = 4). Mice were monitored for 30 days for evidence of tumor recurrence and were subsequently rechallenged with both CT26-KS and CT26 tumor cells on opposite flanks. Proportions represent tumor-free mice after rechallenge.
| Table 3A | ||
|---|---|---|
| Initial Treatment Group | Proportion of tumor-free mice after challenge | |
| CT26-KS | CT26 | |
| Naïve control | 0/4 | 0/4 |
| RFA alone | 5/5 | 1/5 1 |
| huKS-IL2 alone | 3/3 | 3/3 2 |
| RFA + huKS-IL2 | 9/9 | 7/9 3 |
| Table 3B | ||
|---|---|---|
| Proportion of tumor-free mice after challenge | ||
| Initial Treatment Group | CT26-KS1 | CT26 |
| RFA | 3/3 | 1/3 3 |
| Surgery | 6/8 | 0/8 |
| RFA + huKS-IL2 | 4/4 | 3/4 2 |
p= NS vs. control;
p = 0.004 vs. control, p = NS vs. RFA alone;
p = 0.004 vs. control, p = 0.018 vs. RFA alone, p = NS (0.90) vs. huKS-IL2 alone
No statistical difference among all three treatment groups.
p = 0.02 for RFA + huKS-IL2 vs. Surgery, p = NS vs. RFA;
p = NS vs. Surgery
In order to test whether RFA can induce a separate anti-tumor response or simply acts to debulk the tumor to allow IC therapy to be more effective, a series of experiments were designed to compare RFA and IC with complete surgical excision. Mice were implanted with the standard inoculation of CT26-KS tumor cells s.c. in the center of the abdomen. Mice were then treated with complete RFA (40 sec, day 8), complete tumor excision (day 11), or partial RFA (25 sec, day 11) combined with huKS-IL2 on days 11–15. Complete resolution of the primary CT26-KS tumor occurred in all treatment groups (not shown). Mice were monitored for 30 days to document absence of tumor recurrence and then rechallenged with both CT26-KS and CT26 tumor cells on opposite flanks. The results of the tumor rechallenge are presented in Table 3B. Whereas almost all animals rejected the CT26-KS tumor rechallenge, no animals in the surgical excision group rejected the parental CT26 tumor, while 75% of the animals treated with RFA and huKS-IL2 rejected the CT26 tumor. This demonstrated that the combination of RFA plus huKS-IL2 induced a memory repose that was stronger than that induced by surgical excision, and is consistent with the results shown in Table 3A. One of 3 animals treated with RFA alone rejected the CT26 tumor, which was not significant compared to surgical excision. Thus, although the numbers are small, by these criteria (rejection of tumor challenges with CT26-KS and CT26), animals that resolved a primary tumor via RFA show no significant difference from animals that underwent surgical excision of their primary tumor. However, subtle immunological differences were observed when splenocytes from these tumor-free animals were evaluated by flow cytometry (see below).
To determine if the observed immunological memory was tumor-specific, a subset of the animals which rejected both CT26-KS and CT26 in tumor rechallenge experiments and remained tumor-free for an additional 30 days were rechallenged again with CT26-KS (third exposure) and simultaneously injected s.c. with unrelated syngeneic tumor cells (Meth A sarcoma) at a separate site on the opposite flank. Age-matched naive control animals received both CT26-KS and MethA cells in opposite flanks (Table 4). MethA tumors grew progressively in both immune and control animals, whereas CT26-KS tumors were rejected a third time in all immune animals. This demonstrated that the memory response present in immune animals remained specific for the CT26 cell line, as animals which previously rejected both CT26-KS and CT26 could not mount an antitumor response against Meth A.
Table 4.
Tumor-specific memory in mice which rejected CT26-KS tumors and parental CT26 tumors.
Mice which rejected both CT26-KS and CT26 after tumor rechallenge, as described in Table 3, were monitored for tumor recurrence for 30 days. Subsequently, these mice were implanted with 5 × 105 CT26-KS tumor cells (third exposure) or 2×106 MethA sarcoma cells in opposite flanks, to test the tumor specificity of the immune memory response. Age-matched naive mice were similarly inoculated with CT26-KS and MethA cells at the same time. CT26-KS did not grow in any of the immune mice. Data shown are mean ± SEM of 3 mice per group at the conclusion of the experiment (day 18), at which time animals were sacrificed due to MethA tumor size.
| Treatment Group | CT26-KS Tumor Volume (mm3) | Meth A Tumor Volume (mm3) |
|---|---|---|
| Naive mice | 78 ± 2.0 | 760 ± 190 |
| Immune mice | 0.01 | 470 ± 972 |
p=0.0001 for CT26-KS growth in immune mice vs. naive mice.
p=0.24 for Meth A growth in naive mice vs. immune mice.
Treatment with RFA and huKS-IL2 resulted in a stronger T-cell memory response compared to other treatments
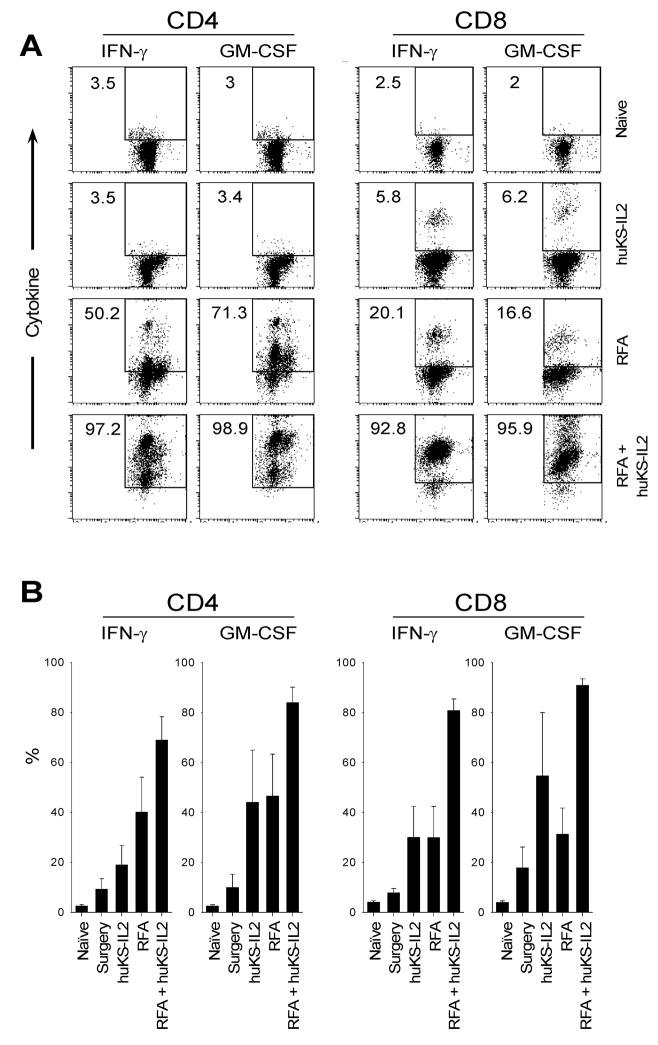
We further evaluated the immune memory response present in animals treated with surgical excision, complete RFA alone, huKS-IL2 alone and partial RFA combined with huKS-IL2 by measuring T-cell cytokine production by flow cytometry. CT26-KS tumor-bearing mice were treated using conditions similar to those used in the tumor rechallenge experiments presented in Tables 3A and 3B so that all animals became tumor-free. All mice were monitored for 30 days to document absence of recurrence, and tumor-free mice were then sacrificed along with age-matched naive control animals. Spleens were harvested and cells were cultured with irradiated CT26-KS or MethA sarcoma cells for 5 days. Viable cells were labeled with markers for CD4 and CD8, as well as intracellular GM-CSF and IFN-γ. A representative experiment is shown in Figure 2A. In this experiment, the combination of RFA and huKS-IL2 IC resulted in greater T-cell responses against CT26-KS cell targets than either RFA or huKS-IL2 IC therapy alone. In the in vitro-stimulated splenocytes from huKS-IL2-treated mice, the proportion of CD4 cells expressing IFN-γ was no different than naive controls (3%). IFN-γ expressing CD4 T cells rose to 50% in the in vitro-stimulated splenocytes from RFA-treated mice; however, 97% of CD4 cells secreted IFN-γ in the in vitro-stimulated splenocytes from mice receiving combination therapy of RFA and huKS-IL2. A similar pattern was seen in CD4 cells with regard to GM-CSF production. The in vitro response to CT26-KS cell targets was even more striking in the CD8 cell population, with only 5–6% of CD8 cells expressing IFN-γ or GM-CSF after huKS-IL2 treatment, 16–20% of cells showing cytokine secretion after RFA treatment, and 92–95% of cells showing cytokine expression after RFA and KS-IL2 combination therapy. There was no significant cytokine expression in spleen cells obtained from either group when stimulated in vitro with MethA cells (data not shown).
Figure 2.
RFA and huKS-IL2 synergize in inducing T cell activation. Splenocytes from animals rendered tumor-free after “complete” RFA, huKS-IL2 IC, complete surgical excision, or combination therapy were followed for 30 days for tumor recurrence.. Mice were sacrificed and splenocytes were isolated, along with age-matched naive animals. Cytokine production was measured via flow cytometry after 5 days of in vitro stimulation with CT26-KS tumor cells. Numbers represent percent CD4 or CD8 positive cells expressing IFN-γ or GM-CSF. A, single representative experiment; B, mean of 3–5 experiments.
These results were reproducible in subsequent analyses. In five flow analyses representing four separate experiments, including mice which underwent complete surgical excision, the proportion of T-cells expressing IFN-γ or GM-CSF after RFA combined with huKS-IL2 continued to be much higher than all other treatment groups. The mean proportions of T-cells expressing cytokines from each experiment are shown in Figure 2B. Since means were calculated from separate individual experiments with different conditions, direct statistical comparison between means could not be calculated.
Discussion
We have shown that growth of CT26-KS colon adenocarcinoma following suboptimal, partial RFA treatment can be significantly impeded and even fully eradicated with the addition of huKS-IL2 IC therapy. Subtherapeutic doses of RFA or IC alone resulted in continued tumor growth without tumor resolution for most animals. Further, there was a significant survival benefit using RFA combined with IC. Treatment with combination therapy affected both treated and untreated distant tumors, and induced a tumor-specific immune memory response not limited to the tumor antigen targeted by the IC. Finally, in vitro analysis using flow cytometry demonstrated that the strongest antitumor T-cell cytokine production occurred after combination therapy.
Our in vivo system used colorectal adenocarcinoma as the target tumor for a number of reasons. First, metastatic colorectal cancer to the liver is very common, and it is the most frequently treated tumor with RFA in many clinical series involving human patients 1,3,9,17,31–33. Roughly 50% of patients with a diagnosis of colon cancer will present with liver metastases or will develop them subsequently 34. Second, survival in patients with untreated metastatic disease is very poor, with a median survival of 6–9 months 4,35. Although resection remains the most effective treatment, only a small number of patients are surgical candidates. Even with a curative resection, the majority of patients will have recurrence in the liver or other systemic sites, 2,9, and although systemic cytotoxic chemotherapy has improved, long term survival has not been impacted 35–37. Finally, EpCAM expression is very common in colorectal adeoncarcinoma which is the target of huKS-IL2 21,23.
RFA, without additional immune stimulation, has been previously shown in both animal and human models to induce an immune response to ablated tissue 28,29. Using a rabbit tumor model, Wissniowski et al. showed that RFA resulted in induction of a local and systemic immune response 28. In patients with hepatocellular carcinoma, T-cells were significantly more responsive to both untreated cultured hepatocellular tumor cells and necrotic tumor one month after RFA 29. However, the presence of an immune response did not correlate clinically with protection from relapse. Although not a major objective of this current study, our data comparing the immune effects of primary tumor elimination by RFA vs. surgical excision are consistent with these prior reports. Namely, RFA treatment appeared to induce a weak T cell memory response as measured in vitro (Figure 2B), not readily seen in animals treated surgically. However, RFA treatment did not result in a substantially stronger memory response to tumor rechallenge than did surgical excision (Table 3B). Augmentation of this weak immune response induced by RFA is likely required for clinical benefit.
The huKS-IL2 immunocytokine previously demonstrated significant preclinical anti-tumor effects as a single agent against EpCAM expressing tumors 20,25,38. As it was substantially more effective than a combination of anti-KS mAb and IL2 (20), we used huKS-IL2, rather than IL2 and antibody alone, as the immunotherapy to combine with RFA. We have previously shown that direct IT injection of huKS-IL2 is more effective than IV administration against palpable tumors. Although IV IC has been shown to localize to the tumor, IT injection facilitates higher local IC concentrations at the tumor and is present for longer periods of time 25. ICs induce both T-cell dependent and independent antitumor effects 20,39. HuKS-IL2 activates both NK cells and T-cells via their IL2 receptor 40,41, as well as Fc receptor (FcR) positive cells such as macrophages, monocytes, neutrophils, and NK cells 24,38,42,43. Both RFA and IC therapy have been shown to independently activate antitumor T-cell responses, and we hypothesized that these two treatments could act synergistically and potentially activate a systemic response against tumor antigens.
Since RFA is not fully curative in clinical practice, our animal model and treatment strategy was developed to simulate a clinical recurrence in patients treated with ablation alone. Therefore, we used “partial RFA”, which caused destruction of the bulk of each treated tumor, but appeared to spare a component of the treated lesion (approximately 25–50% of the viable appearing tumor). Our first series of experiments demonstrated that combination therapy with partial RFA and huKS-IL2 created a synergistic reduction in tumor growth. Both RFA and huKS-IL2 were moderately effective individually compared to untreated animals, but when used in combination, the antitumor response was significantly enhanced. Complete tumor resolution was also demonstrated in some animals. The profound growth suppression resulted in a significant increase in survival in the combination group, which was not limited to the animals which completely resolved the tumor. Ultimately this approach may be targeted for combined treatment of intrahepatic colon cancer metastases. Given the clinical complexity of five daily IT IC treatments to such sites, we also tested the response to a more concentrated dose of IC given in two injections (Fig 1C). When IC was administered alone, the antitumor response was significantly better with five lower dose injections. However, when combined with RFA, tumor growth was not significantly different with two compared to five injections, although there was a trend toward a better response with five IC injections. Further work is needed to create a treatment schedule that would optimize clinical efficacy and applicability.
After observing a synergistic response in tumor growth, our next experiments addressed the ability of RFA or IC to induce immune memory after tumor resolution. The dose of both RFA and huKS-IL2 was increased to create tumor resolution in all treatment groups, including RFA and huKS-IL2 as single agents. All animals which resolved the CT26-KS tumor rejected the CT26-KS tumor upon rechallenge, which is likely related to the high immunogenicity of the human KS epitope on the CT26-KS tumor in mice. However, the parental CT26 colon adenocarcinoma, which is much more aggressive and lacks any human antigens, was also rejected by a majority of animals that were free of CT26-KS due to prior IC treatment. The reactivity against CT26 following huKS-IL2 treatment of the primary CT26-KS tumor confirms prior experiments that demonstrated T-cell activity against CT26 after IV treatment with huKS-IL2 20. Additionally, the antitumor immune response noted in our experiments was tumor specific, as these immune mice did not resist a subsequent challenge with MethA sarcoma. Thus, immune memory was present after either RFA or IC, but induction of effective memory to other antigens expressed on CT26-KS that are shared with the parental CT26 tumor was only present after IC treatment. This result may be clinically significant, as immune memory might help prevent clinical recurrence, and metastatic tumors do not always express the same antigen profile as the primary tumor, so an immune response to several tumor antigens may be important for true clinical cure 38,44–46.
Tumor size is a significant predictor of local recurrence in most clinical RFA series, likely due to incomplete ablation of tumor cells at the tumor margin 4,6,8–11,15,16,32. Larger tumor size has also been correlated with a higher incidence of microsatellite metastases, which result in recurrence and adversely affect survival 47. Although RFA site recurrence is a problem, a much higher percentage of patients recur at distant liver sites or in an extrahepatic location. It is unknown whether local control of hepatic disease by RFA has any impact on systemic disease in human patients 7. Our studies involving mice implanted with two tumors addressed whether the antitumor effect observed with RFA and IC was local or systemic. Interestingly, no animals in the RFA alone group resolved the distant flank tumor, whereas over 50% of mice in the huKS-IL2 group and 81% of animals in the combination group had flank tumor resolution. The previous experiments demonstrate that RFA is primarily a local therapy which can create some immune memory after tumor ablation. However, the addition of IC dramatically increases the systemic antitumor effect. At least 2 mechanisms may be at work on distant tumor sites when IC is given IT in combination with RFA. First, s.c. administered IC, given at sites distant from tumors, can act systemically to mediate IC-induced tumor destruction, similar to that observed with IC given IV (Unpublished observations, Gilles et al, and Yamane et al). Second, the presence of IC in concert with tumor destruction (either immune-mediated or RFA-mediated) might induce a systemic antitumor response against a range of epitopes, enabling activation of tumor-specific effector T cells to circulate systemically and help eradicate distant disease.
Prior studies have used immune therapy to augment the antitumor response after RFA. Den Brok and colleagues treated mice bearing B16 melanoma with a complete ablation and tested tumor-free mice with rechallenge and adoptive transfer 48,49. They found that animals developed a tumor-specific immune response after RFA. Further, this response was transferable to naive mice by IV injection of splenocytes from treated animals, while no immunity was transferred using splenocytes from untreated tumor-bearing animals. In addition, augmentation of the T-cell response using an anti-CTLA-4 antibody enhanced the response to the tumor on rechallenge. This work demonstrated that not just the presence of tumor, but rather RFA specifically induces a T-cell response, and the combination of immune therapy with RFA can significantly enhance the antitumor effect. Our experiments build on this by demonstrating synergy between RFA and IC-related immune therapy, based on local and distant tumor control, detection of tumor-specific memory, and an enhanced response to additional tumor antigen(s) other than the antigenic target directly recognized by the mAb component of the IC.
Future clinical studies may include a combination of chemotherapy, RFA and immune therapy. Many patients included in RFA clinical series received systemic chemotherapy prior to ablation, and neoadjuvant therapy may downsize the tumor to facilitate ablation or resection 3,5,6,15,16. Currently it is not known whether the combination of chemotherapy and RFA is beneficial, but a clinical trial sponsored by the European Organization for Research and Treatment of Cancer (EORTC) has been designed to address this question. Named the CLOCC trial, for Chemotherapy with Local ablation vs. Chemotherapy, it will investigate the effect of ablation with chemotherapy compared to chemotherapy alone 2,6. Our study suggests that adding immune therapy to RFA merits further preclinical development to test whether combining these two treatment modalities could have clinical benefit, either alone or combined with chemotherapy.
This study demonstrates that RFA, which is frequently used clinically, can be improved with the addition of huKS-IL2 immune therapy in this murine model. The potential clinical benefit of adding immunotherapy to RFA is two-fold: to eradicate residual tumor cells at the site of the ablated tumor to potentially reduce local recurrence, and to create a systemic tumor-specific immune response which could eradicate other small tumor deposits elsewhere in the body. The ability of IC to stimulate a polyclonal T-cell response against a variety of tumor antigens may be important in the treatment of metastatic disease. Further work is required to move this potentially effective preclinical therapy to clinical trials. This will include: additional analyses of the mechanisms involved in the synergy observed (e.g., role of T cells, NK cells and FcR), analyses of IC plus RFA regimens in other preclinical tumor models, and testing of combined treatment at more clinically relevant sites, such as hepatic metastases.
Acknowledgments
NIH Grants CA032685, CA87025, CA14520, GM067386, T32-CA090217, The Midwest Athletes for Childhood Cancer Fund, The Crawdaddy Foundation, The UW- Cure Kids Cancer Coalition, and The Super Jake Foundation.
The authors wish to thank Drs. Ann P. O’Rourke, Kenneth Meredith, Jacek Gan, and Jackie Hank for assistance with experimental design and manuscript preparation, Glen E. Leverson, for help with statistical analyses, and Ralph Reisfeld of The Scripps Research Institute for consistent insight, vision, generosity and collaboration.
Footnotes
Statement of Translational Relevance
Radiofrequency ablation is frequently utilized in clinical practice for both primary and metastatic tumors. However, the long term efficacy of ablation is poor due to high rates of both local and systemic recurrence. We sought to enhace the efficacy of ablation by adding immune therapy using the huKS-IL2 immunocytokine. Immunotherapy is most effective in the setting of minimal residual disease, which we utilized in these experiments by adding huKS-IL2 to partially ablated tumors. We show that the immunocytokine was effective in curing partially ablated tumors, and had efficacy against synchronous, untreated tumors at distant sites. This simulates a clinical scenario in which detectable tumor is treated, and a systemic immune response is activated against tumor antigens to resolve distant, undetectable disease. The huKS-IL2 immunocytokine has already completed phase I clinical testing, and since RFA is currently in use, the potential for combination therapy in a clinical setting is very promising.
References
- 1.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Primrose JN. Treatment of colorectal metastases: surgery, cryotherapy, or radiofrequency ablation. Gut. 2002;50:1–5. doi: 10.1136/gut.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood TF, Rose DM, Chung M, Allegra DP, Foshag LJ, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593–600. doi: 10.1007/BF02725339. [DOI] [PubMed] [Google Scholar]
- 4.de Meijer VE, Verhoef C, Kuiper JW, Alwayn IP, Kazemier G, Ijzermans JN. Radiofrequency ablation in patients with primary and secondary hepatic malignancies. J Gastrointest Surg. 2006;10:960–73. doi: 10.1016/j.gassur.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23:1358–64. doi: 10.1200/JCO.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Gillams AR. Liver ablation therapy. Br J Radiol. 2004;77:713–23. doi: 10.1259/bjr/86761907. [DOI] [PubMed] [Google Scholar]
- 7.White RR, Avital I, Sofocleous CT, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg. 2007;11:256–63. doi: 10.1007/s11605-007-0100-8. [DOI] [PubMed] [Google Scholar]
- 8.Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10:338–47. doi: 10.1245/aso.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Siperstein A, Garland A, Engle K, et al. Local recurrence after laparoscopic radiofrequency thermal ablation of hepatic tumors. Ann Surg Oncol. 2000;7:106–13. doi: 10.1007/s10434-000-0106-x. [DOI] [PubMed] [Google Scholar]
- 10.Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633–46. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed M, Goldberg SN. Thermal ablation therapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S231–44. doi: 10.1016/s1051-0443(07)61791-6. [DOI] [PubMed] [Google Scholar]
- 12.Sabel MS, Edge SB. In-situ ablation of breast cancer. Breast Dis. 2001;12:131–40. doi: 10.3233/bd-2001-12113. [DOI] [PubMed] [Google Scholar]
- 13.Pearson AS, Izzo F, Fleming RY, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592–9. doi: 10.1016/s0002-9610(99)00234-2. [DOI] [PubMed] [Google Scholar]
- 14.Chopra S, Dodd GD, III, Chintapalli KN, Leyendecker JR, Karahan OI, Rhim H. Tumor recurrence after radiofrequency thermal ablation of hepatic tumors: spectrum of findings on dual-phase contrast-enhanced CT. AJR Am J Roentgenol. 2001;177:381–7. doi: 10.2214/ajr.177.2.1770381. [DOI] [PubMed] [Google Scholar]
- 15.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–66. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 16.Bowles BJ, Machi J, Limm WM, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg. 2001;136:864–9. doi: 10.1001/archsurg.136.8.864. [DOI] [PubMed] [Google Scholar]
- 17.Higgins H, Berger DL. RFA for liver tumors: does it really work? Oncologist. 2006;11:801–8. doi: 10.1634/theoncologist.11-7-801. [DOI] [PubMed] [Google Scholar]
- 18.Ng KK, Poon RT, Lo CM, Yuen J, Tso WK, Fan ST. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg. 2008;12:183–91. doi: 10.1007/s11605-007-0276-y. [DOI] [PubMed] [Google Scholar]
- 19.Imboden M, Murphy KR, Rakhmilevich AL, et al. The level of MHC class I expression on murine adenocarcinoma can change the antitumor effector mechanism of immunocytokine therapy. Cancer Res. 2001;61:1500–7. [PubMed] [Google Scholar]
- 20.Xiang R, Lode HN, Dolman CS, et al. Elimination of established murine colon carcinoma metastases by antibody-interleukin 2 fusion protein therapy. Cancer Res. 1997;57:4948–55. [PubMed] [Google Scholar]
- 21.Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35:122–8. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Haisma HJ, Pinedo HM, Rijswijk A, et al. Tumor-specific gene transfer via an adenoviral vector targeted to the pan-carcinoma antigen EpCAM. Gene Ther. 1999;6:1469–74. doi: 10.1038/sj.gt.3300969. [DOI] [PubMed] [Google Scholar]
- 23.Mellstedt H, Fagerberg J, Frodin JE, et al. Ga733/EpCAM as a target for passive and active specific immunotherapy in patients with colorectal carcinoma. Ann N Y Acad Sci. 2000;910:254–61. doi: 10.1111/j.1749-6632.2000.tb06713.x. [DOI] [PubMed] [Google Scholar]
- 24.Hank JA, Surfus JE, Gan J, et al. Activation of human effector cells by a tumor reactive recombinant anti-ganglioside GD2 interleukin-2 fusion protein (ch14.18-IL2) Clin Cancer Res. 1996;2:1951–9. [PubMed] [Google Scholar]
- 25.Johnson EE, Lum HD, Rakhmilevich AL, et al. Intratumoral immunocytokine treatment results in enhanced antitumor effects. Cancer Immunol Immunother. 2008;57:1891–902. doi: 10.1007/s00262-008-0519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connor JP, Felder M, Hank J, et al. Ex vivo evaluation of anti-EpCAM immunocytokine huKS-IL2 in ovarian cancer. J Immunother. 2004;27:211–9. doi: 10.1097/00002371-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Ko YJ, Bubley GJ, Weber R, et al. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J Immunother. 2004;27:232–9. doi: 10.1097/00002371-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Wissniowski TT, Hansler J, Neureiter D, et al. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63:6496–500. [PubMed] [Google Scholar]
- 29.Zerbini A, Pilli M, Penna A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139–46. doi: 10.1158/0008-5472.CAN-05-2244. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 31.Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205:367–73. doi: 10.1148/radiology.205.2.9356616. [DOI] [PubMed] [Google Scholar]
- 32.de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175:1619–25. doi: 10.2214/ajr.175.6.1751619. [DOI] [PubMed] [Google Scholar]
- 33.Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–8. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levi F, Lucchini F, Negri E, Boyle P, La Vecchia C. Cancer mortality in Europe, 1995–1999, and an overview of trends since 1960. Int J Cancer. 2004;110:155–69. doi: 10.1002/ijc.20097. [DOI] [PubMed] [Google Scholar]
- 35.Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ. 2000;321:531–5. doi: 10.1136/bmj.321.7260.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 37.Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–47. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 38.Imboden M, Murphy KR, Rakhmilevich AL, et al. The level of MHC class I expression on murine adenocarcinoma can change the antitumor effector mechanism of immunocytokine therapy. Cancer Res. 2001;61:1500–7. [PubMed] [Google Scholar]
- 39.Lode HN, Xiang R, Varki NM, Dolman CS, Gillies SD, Reisfeld RA. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst. 1997;89:1586–94. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 40.Weil-Hillman G, Fisch P, Prieve AF, Sosman JA, Hank JA, Sondel PM. Lymphokine-activated killer activity induced by in vivo interleukin 2 therapy: predominant role for lymphocytes with increased expression of CD2 and leu19 antigens but negative expression of CD16 antigens. Cancer Res. 1989;49:3680–8. [PubMed] [Google Scholar]
- 41.Voss SD, Robb RJ, Weil-Hillman G, et al. Increased expression of the interleukin 2 (IL-2) receptor beta chain (p70) on CD56+ natural killer cells after in vivo IL-2 therapy: p70 expression does not alone predict the level of intermediate affinity IL-2 binding. J Exp Med. 1990;172:1101–14. doi: 10.1084/jem.172.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–25. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 43.Sondel PM, Hank JA. Antibody-directed, effector cell-mediated tumor destruction. Hematol Oncol Clin North Am. 2001;15:703–21. doi: 10.1016/s0889-8588(05)70243-4. [DOI] [PubMed] [Google Scholar]
- 44.Maeurer MJ, Gollin SM, Martin D, et al. Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J Clin Invest. 1996;98:1633–41. doi: 10.1172/JCI118958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon WS, Chang K, Tarnawski AS. Overexpression of metastatic tumor antigen 1 in hepatocellular carcinoma: Relationship to vascular invasion and estrogen receptor-alpha. Hum Pathol. 2004;35:424–9. doi: 10.1016/j.humpath.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Neal ZC, Imboden M, Rakhmilevich AL, et al. NXS2 murine neuroblastomas express increased levels of MHC class I antigens upon recurrence following NK-dependent immunotherapy. Cancer Immunol Immunother. 2004;53:41–52. doi: 10.1007/s00262-003-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103:299–306. doi: 10.1002/cncr.20798. [DOI] [PubMed] [Google Scholar]
- 48.den Brok MH, Sutmuller RP, van d V, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–9. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 49.den Brok MH, Sutmuller RP, Nierkens S, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95:896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]